When it comes to understanding the properties and behavior of acids and bases, acid-base titration curve is a vital concept. It is a graphical representation that illustrates the pH changes that occur during the titration process. As a chemistry enthusiast, it is fascinating to delve into the intricacies of this curve and explore the wealth of information it provides about the strength and concentration of acids and bases.
In this article, we will uncover 20 extraordinary facts about the acid-base titration curve that will enhance your understanding of this fundamental concept. Whether you are a student studying chemistry or a curious individual with a passion for science, these facts will give you a deeper insight into the fascinating world of acid-base titration.
So, grab your laboratory coats and let’s embark on a journey to unravel the secrets behind the acid-base titration curve!
Key Takeaways:
- Acid-base titration curves show how acids and bases react. They help scientists find the right amounts of substances needed for chemical reactions. It’s like a roadmap for mixing the perfect ingredients in a recipe!
- By studying titration curves, scientists can figure out the strength and concentration of acids and bases. It’s like using a special map to discover the secrets of different chemicals!
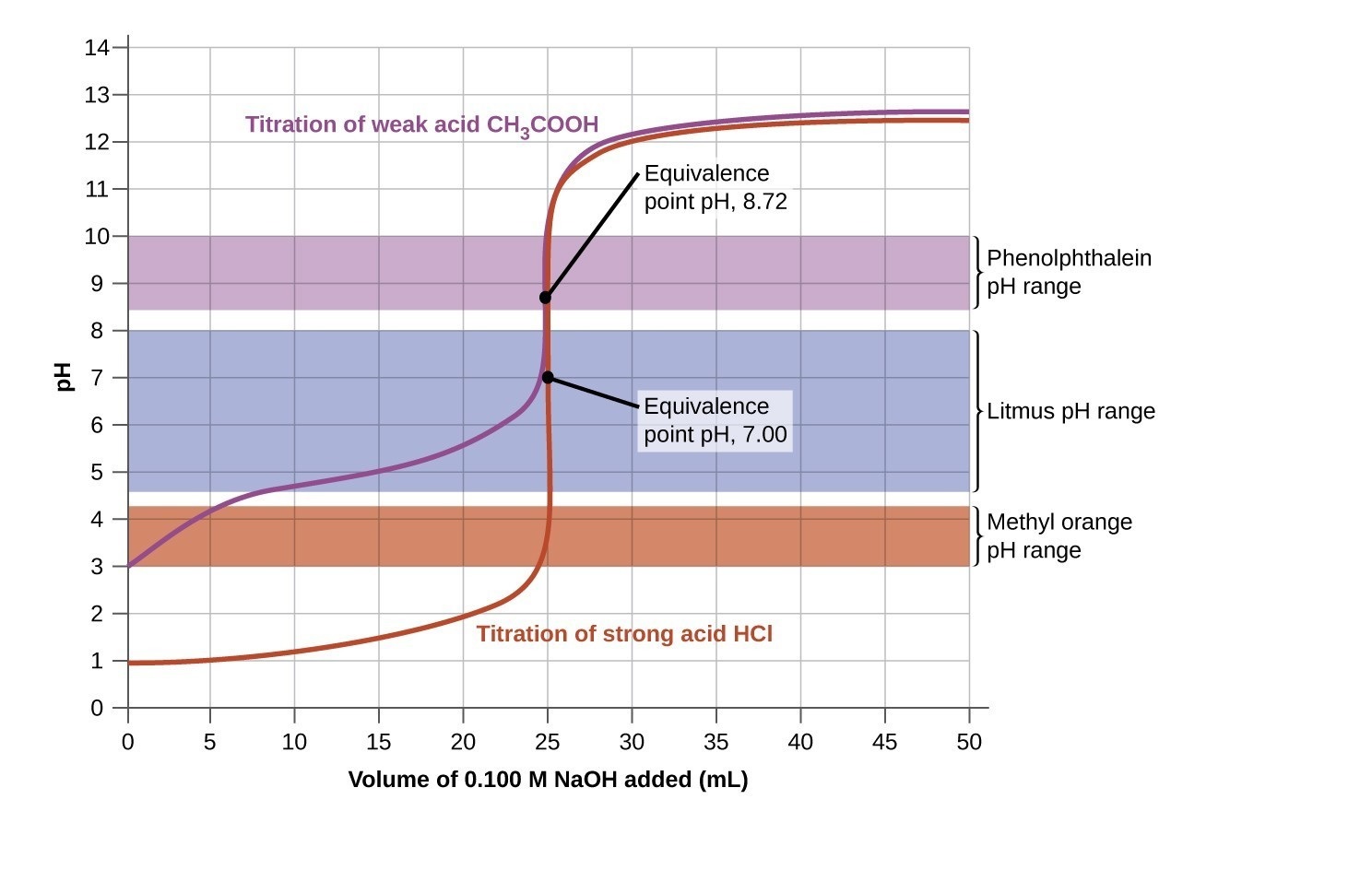
The acid-base titration curve is a graphical representation.
The curve illustrates the pH changes that occur during the titration of an acid with a base or vice versa. It plots pH on the y-axis against the volume of added titrant on the x-axis.
It provides valuable information about the equivalence point.
The equivalence point is the point at which the moles of acid equals the moles of base in a chemical reaction. The titration curve helps identify this crucial point.
The equivalence point appears as a sharp vertical rise on the titration curve.
During the equivalence point, a small amount of added titrant causes a significant change in pH due to the complete neutralization of the acid or base being titrated.
The steepest slope of the curve occurs around the equivalence point.
This steep slope indicates a rapid change in pH, which is attributed to the neutralization reaction being close to completion.
The initial pH depends on the strength of the acid or base being titrated.
Strong acids or bases have lower initial pH values, whereas weak acids or bases have higher initial pH values.
The pH at the halfway point of titration is known as the half-equivalence point.
At this point, half of the acid or base has been neutralized, resulting in a pH that is halfway between the initial pH and the equivalence point.
Buffers can influence the shape of the titration curve.
If a buffer solution is present, it can resist changes in pH and result in a more gradual slope around the equivalence point.
The shape of the curve can be affected by temperature.
Increasing the temperature can alter the dissociation of the acid or base being titrated, leading to variations in the curve’s shape.
Different indicators can be used to visualize the titration curve.
Indicators change color at specific pH ranges, allowing for the detection of the equivalence point. Examples include phenolphthalein, bromothymol blue, and methyl orange.
Titration curves are essential in determining the concentration of acids and bases.
By analyzing the curve, scientists can calculate the concentration of unknown solutions based on known volumes and molarities of the titrant.
Complex acid-base titration curves can involve multiple equivalence points.
Some reactions contain multiple acidic or basic species, resulting in multiple equivalence points and more intricate curves.
Acid-base titration curves can also be used to study weak acids and bases.
In the case of weak acids or bases, the curve displays a gradual slope and a less distinct equivalence point.
The pKa or pKb values of weak acids or bases can be determined from the titration curve.
By analyzing the pH at the half-equivalence point, the pKa or pKb values, which indicate the strength of weak acids or bases, can be calculated.
The choice of titration method can affect the shape of the curve.
Titration methods such as acid-base, redox, and complexometric titrations can lead to different curve profiles and characteristics.
Potentiometric titration can provide precise pH measurements during the titration process.
This method involves using a pH electrode connected to a pH meter to continuously monitor and record changes in pH, resulting in a more accurate titration curve.
Acid-base titration curves are used extensively in the pharmaceutical industry.
Pharmaceutical scientists rely on these curves to determine the concentration of active ingredients in medications and assess their stability and efficacy.
The steepness of the curve is influenced by the concentration of the acid or base being titrated.
Higher concentrations lead to steeper curves, while lower concentrations result in more gradual curves.
Acid-base titration curves can be influenced by the presence of impurities.
Impurities in the acid or base can alter the curve, making it important to ensure high-quality reagents for accurate results.
The titration curve can be used to determine the pH of a solution at any point during the titration.
By referring to the curve, scientists can identify the pH value corresponding to any volume of added titrant.
Acid-base titration curves are fundamental to understanding acid-base reactions and their stoichiometry.
By studying these curves, scientists gain insights into the proportions of reactants and products involved in acid-base reactions.
These 20 extraordinary facts about acid-base titration curves demonstrate their significance in understanding the nature of acids and bases and their interactions. Whether in laboratories, industries, or pharmaceutical research, titration curves play a vital role in chemical analysis and contribute to advancements in diverse fields.
Conclusion
In conclusion, the acid-base titration curve is a fascinating topic in the field of chemistry. Understanding how pH changes during a titration can provide valuable insights into the nature of acids and bases and their reaction with one another. The curve provides important information about the equivalence point, buffering capacity, and the presence of any additional dissociation or hydrolysis reactions that may occur.
By analyzing the curve, scientists can determine the concentration of an unknown acid or base, identify the identity of an unknown compound, and even discover the presence of impurities in a sample. It is a powerful tool that is widely used in many scientific disciplines, including analytical chemistry, environmental science, and biochemistry.
Studying the acid-base titration curve offers endless possibilities for discovery and innovation, and it continues to shape our understanding of chemical reactions. By delving deeper into the intricate details of this process, we can unlock new insights that have the potential to revolutionize various industries and improve our understanding of the world around us.
FAQs
1. What is an acid-base titration curve?
An acid-base titration curve is a graphical representation of how the pH of a solution changes during the process of titration, where an acid reacts with a base or vice versa.
2. What information can we obtain from an acid-base titration curve?
The acid-base titration curve provides information about the equivalence point, buffering capacity, and the presence of any additional dissociation or hydrolysis reactions that may occur. It can be used to determine the concentration of unknown acids or bases, identify unknown compounds, and detect impurities in a sample.
3. How is the pH value determined during a titration?
The pH value is determined using a pH indicator, such as litmus paper or a pH meter. The indicator changes color when the solution reaches a specific pH, indicating the completion of the titration.
4. What factors can influence the shape of an acid-base titration curve?
The shape of the curve can be influenced by the strength and concentration of the acid and base, the nature of the reactants, the presence of other compounds, the temperature of the solution, and the volume of titrant added.
5. How is an acid-base titration curve useful in different industries?
The acid-base titration curve is widely used in various industries, including pharmaceuticals, food and beverage, environmental science, and quality control. It helps determine the purity of substances, assess the effectiveness of chemical reactions, and ensure the proper functioning of products and processes.
Acid-base titration curves provide valuable insights into chemical reactions, but there's more to explore in the world of chemistry. Uncover the secrets of buffer solutions and their role in maintaining stable pH levels. Dive into fascinating chemistry facts that will ignite your curiosity and expand your knowledge. For those interested in the analytical side, discover captivating facts about analytical chemistry and its real-world applications.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
