Acids and bases are fundamental concepts in the field of chemistry, playing crucial roles in various natural and industrial processes. Understanding the properties and behaviors of acids and bases is not only essential for scientific research and innovation but also for everyday applications, ranging from household cleaning products to pharmaceuticals. In this article, we'll delve into 20 intriguing facts about acids and bases, shedding light on their significance and impact in the world around us. From the origins of the term "acid" to the diverse uses of bases in different industries, we'll explore the fascinating characteristics and applications of these chemical entities. Whether you're a science enthusiast, a student seeking to expand your knowledge, or simply curious about the substances that shape our environment, this exploration of acids and bases promises to unveil a wealth of captivating insights. So, let's embark on a journey through the realm of chemistry and uncover the compelling facts that make acids and bases integral components of our lives.
Key Takeaways:
- Acids and bases are everywhere, from the food we eat to the products we use, shaping our daily lives and playing a crucial role in chemistry, industry, and healthcare.
- The pH scale measures acidity and basicity, with acids and bases impacting everything from the tangy flavor of lemon juice to the production of high-quality paper products.
Acids and bases are found everywhere.
From the food we eat to the products we use, acids and bases play a crucial role in our daily lives. They are present in fruits, cleaning products, and even the human body, underscoring their pervasive influence.
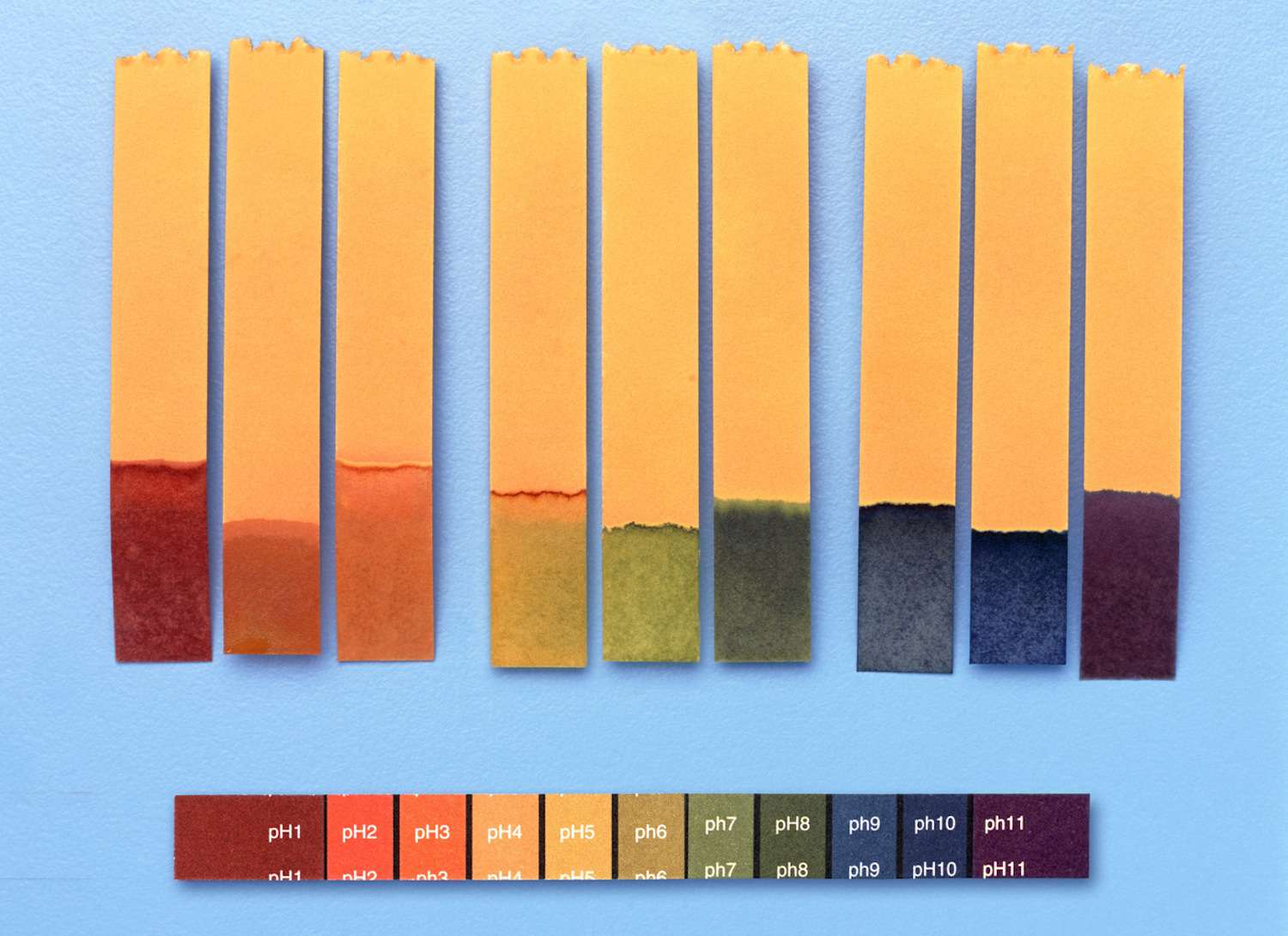
The pH scale measures acidity and basicity.
The pH scale, ranging from 0 to 14, quantifies the acidity or basicity of a substance. A pH of 7 is considered neutral, while values below 7 indicate acidity and those above 7 denote basicity.
Lemon juice is acidic.
The tangy flavor of lemon juice stems from its acidic nature, primarily due to the presence of citric acid. This acidity contributes to its versatile culinary uses and preservation properties.
Bases can feel slippery.
When in contact with bases, such as soaps or household cleaners, a slippery sensation is often experienced. This tactile characteristic is a result of the reaction between the base and oils on the skin.
Acids and bases conduct electricity.
In aqueous solutions, acids and bases can conduct electricity due to the presence of ions. This property is harnessed in various applications, including battery technology and chemical synthesis.
Bases neutralize acids.
Through a chemical reaction called neutralization, bases can counteract the effects of acids, leading to the formation of water and a salt. This process is integral in various industrial and laboratory settings.
The stomach contains hydrochloric acid.
Hydrochloric acid, a potent acid with a pH of approximately 2, aids in digestion by breaking down food and killing harmful bacteria in the stomach. This acidic environment is crucial for maintaining digestive health.
Bases are essential in household cleaning.
Many household cleaning agents, such as ammonia and baking soda, are classified as bases. Their ability to emulsify oils and dissolve grime makes them indispensable for maintaining a clean and hygienic living space.
Acids can react with metals.
Certain acids, like hydrochloric acid, exhibit a vigorous reaction when in contact with reactive metals such as zinc or magnesium. This reaction releases hydrogen gas and dissolves the metal, showcasing the corrosive nature of acids.
Bases are used in agriculture.
In agriculture, bases like lime are employed to neutralize acidic soils, thus enhancing the fertility and productivity of the land. This application underscores the pivotal role of bases in promoting sustainable farming practices.
Acid rain has detrimental effects.
The infiltration of acidic precipitation, known as acid rain, can have adverse effects on the environment, including the acidification of bodies of water and damage to vegetation and infrastructure.
Bases play a role in pharmaceuticals.
Many medications are formulated as salts of basic compounds to enhance their stability and solubility. This utilization of bases in pharmaceuticals ensures the efficacy and safety of various medicinal products.
Acids are crucial in food preservation.
Acids, such as vinegar and citric acid, are utilized in preserving food through processes like pickling and canning. Their antimicrobial properties inhibit the growth of spoilage microorganisms, extending the shelf life of perishable items.
Bases are integral in wastewater treatment.
In wastewater treatment facilities, bases like sodium hydroxide are employed to adjust the pH of effluents, precipitate metals, and neutralize acidic waste streams, contributing to environmental protection and resource conservation.
Acids and bases are central to titrations.
Titrations, a common analytical technique, rely on the reaction between acids and bases to determine the concentration of a solution. This method is widely utilized in laboratory experiments and quality control processes.
Bases are used in the production of paper.
In papermaking, bases like sodium hydroxide are utilized to break down lignin in wood pulp, facilitating the separation of fibers and the production of high-quality paper products.
Acids and bases play a role in skincare.
The pH of skincare products, including cleansers and exfoliants, is carefully formulated to maintain the skin's natural acid mantle. This balance is crucial for skin health and the prevention of irritation and dryness.
Bases are crucial in the synthesis of polymers.
The production of various polymers, including nylon and polyester, involves the use of basic catalysts and reagents. Bases contribute to the synthesis and processing of polymers with diverse industrial applications.
Acids and bases are central to chemical equilibrium.
In chemical reactions, acids and bases play a pivotal role in establishing and maintaining equilibrium. Their interactions influence reaction rates and product formation, driving numerous chemical processes.
Conclusion
In conclusion, understanding the properties and behaviors of acids and bases is crucial in various scientific fields and everyday life. From the unique chemical reactions they undergo to their influence on the environment and human health, acids and bases play a significant role in shaping the world around us. By delving into the 20 fascinating facts about acids and bases, we gain a deeper appreciation for their impact and the intricate balance they maintain. Whether it's the sour taste of lemons, the fizz of carbonated drinks, or the importance of pH levels in our bodies, the ubiquity of acids and bases underscores their importance. As we continue to explore and comprehend these fundamental substances, we uncover new insights that can lead to innovative solutions and advancements across diverse industries.
FAQs
What are some common examples of acids and bases?Acids are commonly found in citrus fruits like lemons and oranges, as well as in vinegar and stomach acid. On the other hand, bases can be found in household items such as baking soda, antacids, and soaps.
How do acids and bases affect the environment?Acid rain, caused by sulfuric and nitric acids, can harm the environment by damaging forests, lakes, and aquatic life. Bases, when released into water bodies, can raise the pH levels, impacting aquatic ecosystems and marine life.
Acids and bases are truly fascinating, but there's still more to explore! For a lighthearted look at these essential compounds, check out our fun facts article. If you're curious about the HSAB theory and how it predicts chemical reactions, our astonishing facts piece will satisfy your curiosity. And for those interested in the practical applications of acid-base chemistry, our captivating article on titration is a must-read. Each of these articles offers a unique perspective on the world of acids and bases, so dive in and expand your knowledge!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
