An acid-base buffer is a crucial concept in chemistry, playing a significant role in maintaining the pH balance of substances. It refers to a solution that resists changes in pH when an acid or base is added to it. Buffer systems are vital in various biological and chemical processes, making them a fascinating topic to explore.
In this article, we will delve into 19 captivating facts about acid-base buffers that will broaden your understanding of their importance and functionality. Whether you are a chemistry enthusiast or simply curious about the inner workings of this chemical phenomenon, these facts are sure to engage and educate.
So, let’s embark on this journey of discovery as we unravel the world of acid-base buffers and learn about their fascinating properties, applications, and impact on our daily lives.
Key Takeaways:
- Acid-base buffers help maintain a stable pH level in the body and the environment, ensuring optimal functioning of biological systems and preventing harmful acidification.
- Acid-base buffers are like the superheroes of chemistry, maintaining the right pH in our bodies, food, and even swimming pools. They resist changes in acidity and keep everything in balance.
What is an Acid-Base Buffer?
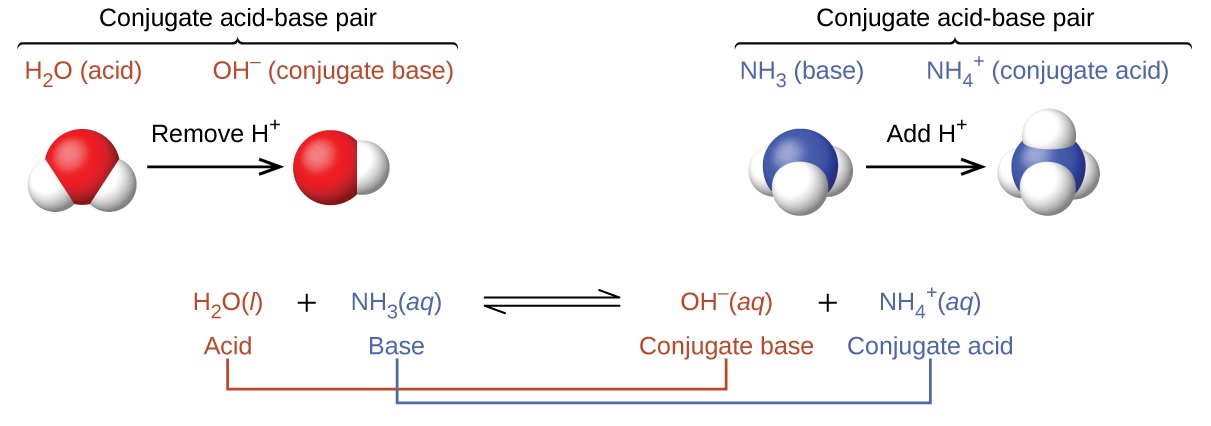
An acid-base buffer is a solution that helps maintain a stable pH level by resisting changes in acidity or alkalinity. It consists of a weak acid and its conjugate base, or a weak base and its conjugate acid.
How do Acid-Base Buffers Work?
Acid-base buffers work through a process called neutralization, where the acids and bases in the solution react with each other to form water and a salt. This reaction helps to maintain the pH level within a certain range.
Importance of Acid-Base Buffers in Biological Systems
Acid-base buffers are crucial in maintaining the proper pH balance in various biological systems, including the blood, cells, and tissues. They ensure that essential biochemical reactions can occur optimally.
Buffer Capacity
Buffer capacity is a measure of the ability of an acid-base buffer solution to resist changes in pH. It depends on the concentration of the buffer components and their relative strengths.
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation is a mathematical expression that relates the pH of a buffer solution to the pKa (acid dissociation constant) of the weak acid and the ratio of the conjugate base to weak acid concentrations.
Common Examples of Acid-Base Buffers
Some common examples of acid-base buffers include the bicarbonate buffer system in the blood, the phosphate buffer system in cells, and the acetic acid/acetate buffer system used in laboratories.
Acid-Base Buffer in Antacids
Many antacids contain acid-base buffers to help neutralize excess stomach acid. These buffers, such as aluminum hydroxide or magnesium hydroxide, provide quick relief from symptoms of acid reflux and indigestion.
Role of Acid-Base Buffers in the Environment
Acid-base buffers play a crucial role in maintaining the pH balance in natural environments, such as lakes, rivers, and soil. They help prevent harmful acidification and keep ecosystems functioning properly.
Buffer Solutions in Chemistry Experiments
In chemistry experiments, buffer solutions are often used to maintain a constant pH level during reactions. This ensures accurate and reproducible results, especially when working with sensitive or pH-dependent substances.
Acid-Base Buffer in the Food Industry
Acid-base buffers are widely used in the food industry to control the pH of various products. They help maintain the desired taste, texture, and shelf life of food and beverages.
Buffering Action of Baking Soda
Baking soda, or sodium bicarbonate, is a versatile acid-base buffer commonly used in baking. It reacts with acidic ingredients, releasing carbon dioxide gas and causing the dough to rise.
Buffering Capacity of Biological Fluids
Biological fluids like blood and intracellular fluid have high buffering capacities to maintain stable pH levels. This is essential for the proper functioning of enzymes, proteins, and other biomolecules.
Acid-Base Balance in the Human Body
The acid-base balance in the human body is tightly regulated through various mechanisms, including the respiratory system, kidneys, and buffers. This ensures optimal physiological functioning and homeostasis.
Buffering Role of Carbonic Acid and Bicarbonate
In the bicarbonate buffer system, carbonic acid (H2CO3) acts as a weak acid, while bicarbonate ions (HCO3-) act as its conjugate base. Together, they help maintain the pH balance in the blood.
pH Range of Acid-Base Buffers
Acid-base buffers are most effective within a specific pH range, known as the buffer range. This range typically lies within one unit above and below the pKa of the weak acid in the buffer system.
Acid-Base Buffer in Pharmaceutical Formulations
Buffering agents are often added to pharmaceutical formulations to maintain the stability and efficacy of drugs. These buffers help prevent degradation and enhance the absorption of orally administered medications.
Buffering Action in Swimming Pools
Acid-base buffers are employed in the swimming pool industry to maintain the pH balance of the water. This ensures a safe and comfortable swimming environment while preventing corrosion of pool equipment.
Buffer Solutions in Analytical Chemistry
Buffer solutions are extensively used in analytical chemistry for calibration and standardization purposes. They provide a known pH level, allowing accurate measurement and analysis of various substances.
Buffer Capacity and Titration
Buffer solutions with high buffer capacity are often used in acid-base titrations to maintain a steady pH during the addition of titrant. This enables accurate determination of the unknown concentration of the analyte.
Conclusion
Acid-base buffers play a crucial role in maintaining the pH balance in our bodies and various chemical processes. These remarkable substances have unique properties that allow them to resist changes in pH when acids or bases are added. Hopefully, this article has shed light on the fascinating world of acid-base buffers and provided you with a deeper understanding of their importance in chemistry and our everyday lives.
FAQs
Q: What is an acid-base buffer?
An acid-base buffer is a solution that can resist changes in pH when an acid or a base is added. It is made up of a weak acid and its conjugate base, or a weak base and its conjugate acid.
Q: How do acid-base buffers work?
Acid-base buffers work by utilizing the principles of equilibrium. When an acid is added, the buffer’s weak base component reacts with the acid to neutralize it. Similarly, when a base is added, the buffer’s weak acid component reacts with the base to neutralize it. This keeps the pH of the solution relatively stable.
Q: What are some examples of acid-base buffers?
Some common examples of acid-base buffers include the bicarbonate ion (HCO3-) in the blood, which helps regulate pH, and the phosphate buffer system found in cells and the extracellular fluid.
Q: What is the importance of acid-base buffers?
Acid-base buffers are essential for maintaining the pH balance in living systems. They play a crucial role in various biological processes, such as enzyme activity, cellular respiration, and the proper functioning of organs and systems.
Q: Can acid-base buffers be found in nature?
Absolutely! Acid-base buffers are found throughout nature. For example, the carbonate buffer system helps regulate the pH in the oceans, preventing drastic changes that could harm marine life.
Q: Can I create my own acid-base buffer?
Yes, it is possible to create your own acid-base buffer by combining a weak acid and its conjugate base or a weak base and its conjugate acid. This allows you to control the pH of a solution and maintain it within a desired range.
Acid-base buffers play a crucial role in maintaining stable pH levels, but that's just the beginning. Dive deeper into the world of chemistry and explore how blood buffers help regulate <pH balance|20 Fascinating Facts About Blood Buffer> in our bodies. Uncover the secrets behind <acid-base reactions|18 Enigmatic Facts About Bronsted-Lowry Acid-Base Theory> and their significance in various chemical processes. And if you're curious about the art of <titration|14 Mind-blowing Facts About Titration>, prepare to have your mind blown by the fascinating facts that await you.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.