When it comes to understanding the interactions between acids and bases, the Hard-Soft Acid-Base (HSAB) theory plays a crucial role. Developed by Ralph Pearson in the 1960s, this theory provides a framework for explaining chemical reactions based on the relative hardness or softness of the reacting species.
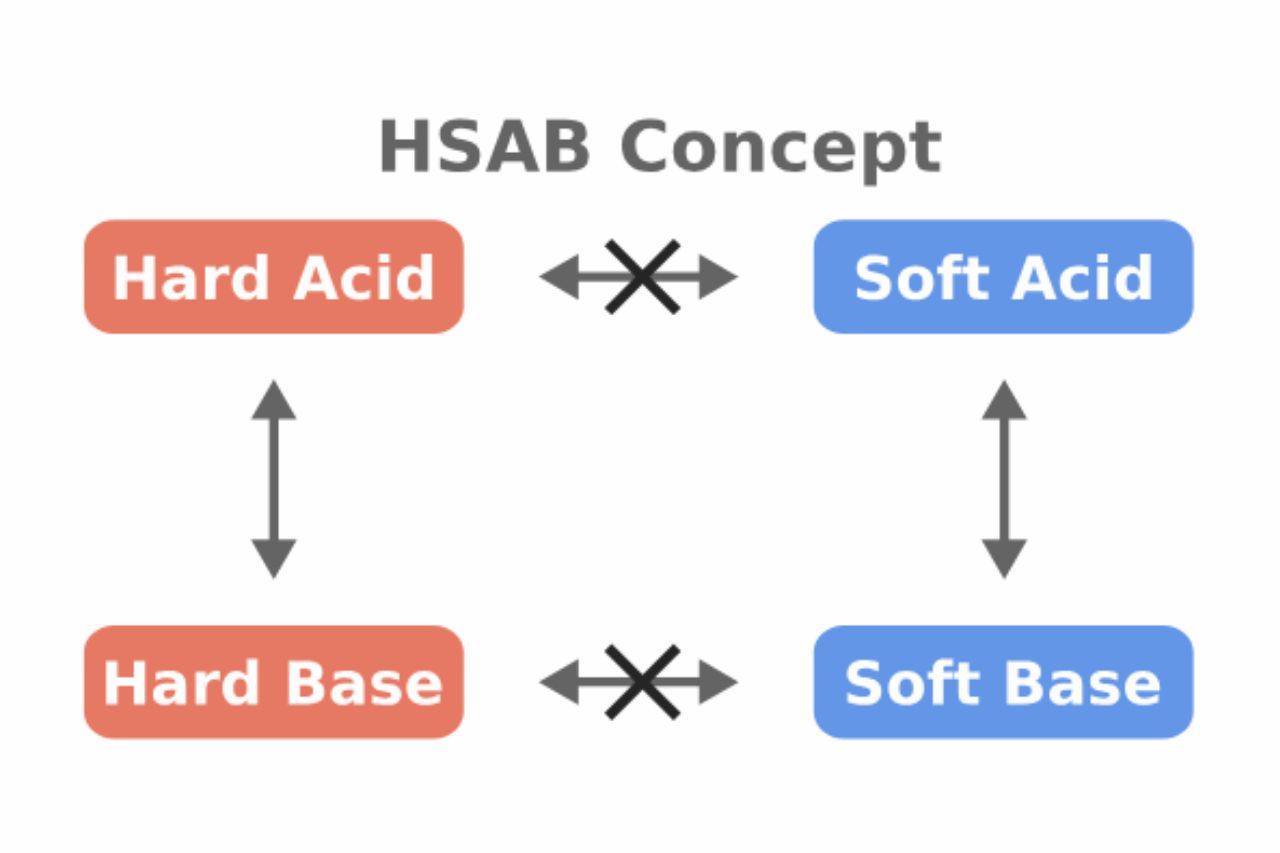
The HSAB theory categorizes acids and bases into two groups: hard and soft. Hard acids are typically small, highly charged ions with little polarizability, while soft acids are larger ions with lower charge density. On the other hand, hard bases are generally small, electron-deficient molecules, while soft bases are larger with high electron density.
In this article, we will explore 15 astonishing facts about the Hard-Soft Acid-Base theory that will enhance your understanding of chemical reactions and the properties of acids and bases. So, let’s dive into the fascinating world of HSAB theory!
Key Takeaways:
- The HSAB Theory helps chemists understand how different acids and bases interact based on their electronic structure, guiding the design of new materials and chemical processes.
- By recognizing the preferences of hard and soft acids and bases, the HSAB Theory allows chemists to predict reactivity and understand complex chemical reactions in fields like biochemistry and materials science.
Origins of the HSAB Theory
The HSAB Theory was first introduced by Ralph Pearson in the 1960s. It has since become one of the most influential theories in inorganic chemistry.
Hard Acids and Bases
In the HSAB Theory, hard acids are characterized by having high charge density and low polarizability. Examples include alkali metal cations and small transition metal ions.
Soft Acids and Bases
On the other hand, soft acids have low charge density and high polarizability. Examples are noble gas atoms, large transition metals, and heavy metal ions.
Acid-Base Interactions
The HSAB Theory states that hard acids prefer to react with hard bases, while soft acids tend to react with soft bases. This preference is based on the principle of maximizing stability in the resulting complexes.
Geometric Considerations
The theory also takes into account the geometry of the acid-base interaction. Hard acids and bases tend to form more linear complexes, while soft acids and bases favor more covalent, three-dimensional structures.
Solvent Effects
Solvent plays a crucial role in the HSAB Theory. Hard acids and bases have a higher affinity for polar solvents, while soft acids and bases are more likely to interact favorably with nonpolar solvents.
Preference of Hard Bases
Hard bases have a greater tendency to form bonds with metal ions due to their stronger electrostatic interactions. This makes them particularly important in biological systems where metal ions are abundant.
Inorganic Chemistry Applications
The HSAB Theory is widely used in inorganic chemistry to explain and predict the stability and reactivity of various metal complexes.
Organic Chemistry Application
In organic chemistry, the HSAB Theory is employed to understand reaction mechanisms, especially those involving Lewis acid-base interactions.
Industrial Applications
The HSAB Theory is used in various industries, including pharmaceuticals, materials science, and catalysis, to design and optimize chemical processes and develop new materials.
Transition Metal Chemistry
The HSAB Theory provides valuable insights into the behavior of transition metal ions and their complex formation, making it a fundamental concept in transition metal chemistry.
Biochemical Relevance
The HSAB Theory is essential for understanding coordination chemistry in biological systems, as metal ions often play critical roles in enzymatic reactions and other biochemical processes.
Predicting Reactivity
By applying the HSAB Theory, chemists can make predictions about the reactivity of different acid-base pairs, aiding in the design of more efficient synthetic routes and minimizing unwanted side reactions.
Limitations of the HSAB Theory
While the HSAB Theory is highly useful, it cannot account for all acid-base interactions, and exceptions to its predictions do exist. Nonetheless, it provides a valuable framework for understanding many chemical processes.
Continued Research
The HSAB Theory continues to be an active area of research, with scientists exploring its applications in new areas and refining its concepts to improve its precision and effectiveness.
Conclusion
Hard-Soft Acid-Base (HSAB) theory is a fundamental concept in chemistry that provides insight into the reactions and interactions between acids and bases. By categorizing acids and bases as “hard” or “soft” based on their electronic and molecular properties, the theory offers a predictive framework for understanding chemical reactions.
This theory has several implications in various fields of chemistry, including organic chemistry, inorganic chemistry, and biochemistry. It helps chemists elucidate reaction mechanisms, design catalysts, predict solubilities, and understand fundamental concepts in chemical bonding.
By recognizing that hard acids prefer to react with hard bases, while soft acids have a propensity to react with soft bases, the HSAB theory allows chemists to predict the feasibility of different reactions and the formation of stable complexes. This understanding is crucial in fields such as drug design, materials science, and industrial chemistry, where controlling the reactivity and selectivity of chemical reactions is critical.
In summary, the Hard-Soft Acid-Base theory is a powerful tool that enhances our understanding of chemical interactions and provides a basis for predicting and controlling chemical reactions. Its broad applications make it a cornerstone of modern chemistry.
FAQs
Q: What is the Hard-Soft Acid-Base theory?
A: The Hard-Soft Acid-Base (HSAB) theory categorizes acids and bases as “hard” or “soft” based on their electronic and molecular properties. It explains how these different acid-base pairs interact and react with each other.
Q: How does the HSAB theory help in understanding chemical reactions?
A: The HSAB theory allows chemists to predict the reactivity and stability of chemical reactions by determining which combinations of hard acids and hard bases, or soft acids and soft bases, are more likely to form stable complexes.
Q: What are some practical applications of the HSAB theory?
A: The HSAB theory finds applications in various fields such as drug design, materials science, and industrial chemistry. It helps in designing efficient catalysts, predicting solubility, understanding reaction mechanisms, and controlling chemical selectivity.
Q: Can the HSAB theory be applied to organic chemistry?
A: Yes, the HSAB theory is applicable to organic chemistry. It aids in understanding acidity and basicity of organic compounds, predicting reactivity in organic reactions, and designing organic catalysts.
Q: Is the HSAB theory universally accepted?
A: The HSAB theory is widely accepted and has been validated by numerous experimental observations. However, like any scientific theory, it is continuously evolving and subject to refinement as new evidence and insights emerge.
Exploring the fascinating world of chemistry? Dive deeper into <acid-base reactions> with our exploration of Bronsted-Lowry Acid-Base Theory. Uncover the secrets of <coordination chemistry> and its wide-ranging applications. Don't miss our revelations about <chemical reactivity> and periodic trends that shape the behavior of elements. Chemistry enthusiasts won't want to miss these engaging articles!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
