Coordination chemistry is a fascinating branch of chemistry that deals with the study of compounds formed by the interaction of metal ions and ligands. These compounds, known as coordination complexes, have unique properties and play a crucial role in various fields such as materials science, catalysis, bioinorganic chemistry, and medicine. The study of coordination chemistry allows chemists to understand the processes involved in the formation, structure, and reactivity of these complexes.
In this article, we will explore nine astounding facts about coordination chemistry that will enhance your understanding of this captivating field. From the diverse range of ligands to the intricate geometries exhibited by coordination complexes, each fact sheds light on the complexity and significance of coordination chemistry in the world of science.
Key Takeaways:
- Ligands are like matchmakers for metal atoms, creating colorful and diverse coordination complexes with unique properties that can be used in medicine, industry, and environmental protection.
- Transition metal complexes are like chemical superheroes, using their catalytic powers to speed up reactions and fight diseases, while also helping to clean up the environment by removing toxins from water.
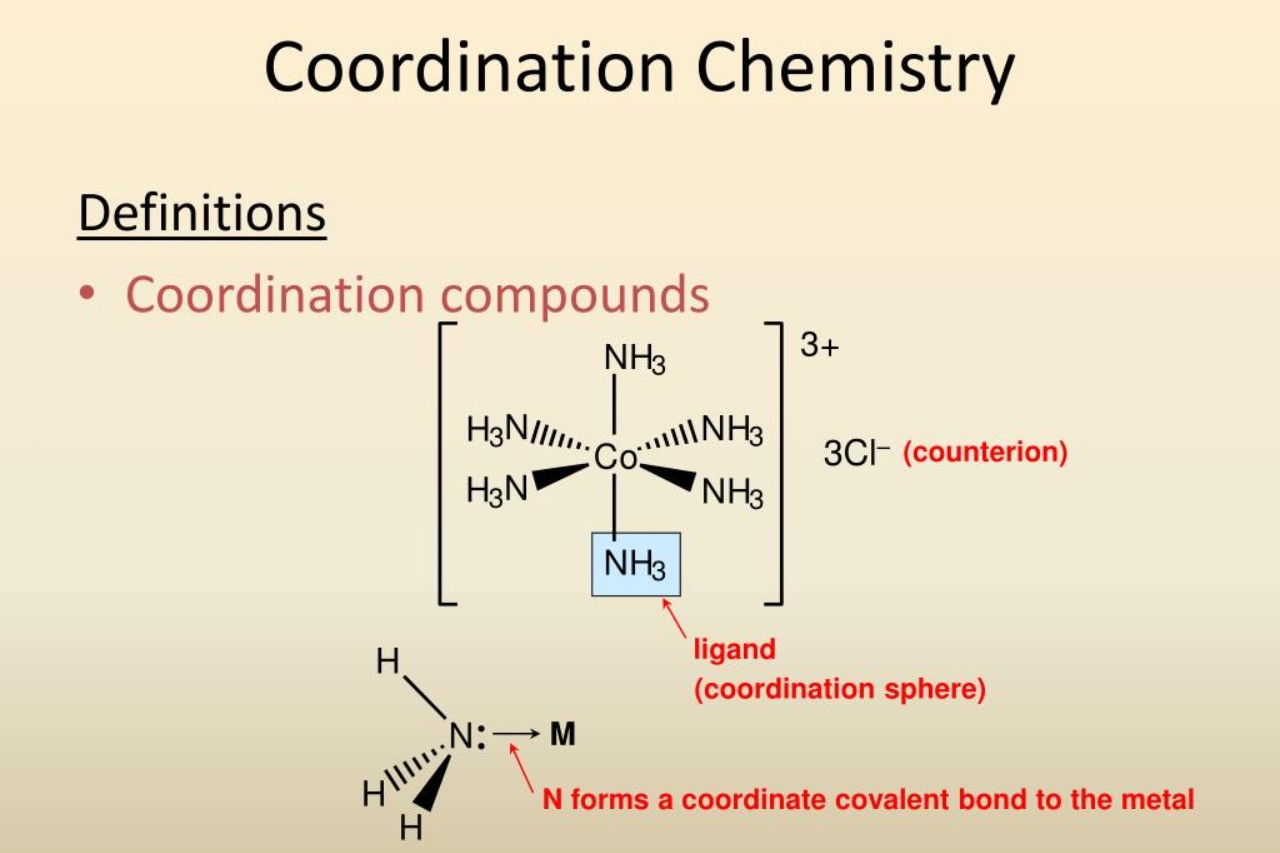
The Role of a Ligand
In coordination chemistry, one of the most fascinating aspects is the role played by ligands. Ligands are molecules or ions that bind to a central metal atom or ion to form a coordination complex. They provide a crucial framework for the coordination chemistry to occur by donating electrons to the metal center.
Coordination Numbers
Each coordination complex has a coordination number, which represents the number of ligands surrounding the central metal atom or ion. Coordination numbers can vary greatly, ranging from 2 to 12 or even higher. The coordination number greatly influences the physical and chemical properties of the complex.
Transition Metal Complexes
Coordination chemistry predominantly involves transition metal complexes. Transition metals are known for their ability to form stable coordination bonds with ligands due to the availability of d orbitals in their valence shell. This property allows them to exhibit a wide range of colors and magnetic properties.
Chelation
Chelation involves the formation of a complex with a ligand that binds to the metal ion through multiple donor atoms. This creates a ring-like structure called a chelate. Chelation is widely used in various applications, such as medicine, industrial processes, and environmental sciences.
Isomerism
Coordination compounds often exhibit different forms of isomerism. This includes structural isomerism (where the connectivity of atoms in the complex differs) and stereochemical isomerism (where the spatial arrangement of ligands around the metal ion is different). Isomerism adds to the complexity and diversity of coordination chemistry.
Catalytic Properties
Many coordination compounds, particularly transition metal complexes, possess remarkable catalytic properties. They can accelerate chemical reactions by providing an alternative reaction pathway with lower energy barriers. This has significant implications in various industrial processes and biological systems.
Colorful Complexes
Coordination compounds are often vividly colored. This phenomenon arises from the absorption of light by the complex, causing certain wavelengths to be reflected or transmitted, resulting in the observed color. The color of a complex is highly dependent on the nature of the ligands and the metal center.
Applications in Medicine
Coordination chemistry plays a vital role in medicinal chemistry and drug design. Many metal-based coordination complexes exhibit promising properties for the treatment of various diseases, including cancer. They can selectively target cancer cells, deliver drugs efficiently, and enhance therapeutic efficacy.
Environmental Significance
Coordination chemistry also finds applications in environmental sciences. Metal complexes can be utilized for the removal of toxins from contaminated water, as catalysts in environmental remediation processes, or in the detection and sensing of pollutants. The study of coordination chemistry contributes to developing sustainable solutions to global environmental challenges.
Conclusion
Coordination chemistry is a fascinating field that explores the interactions between metal ions and ligands. These interactions give rise to a wide range of compounds with unique properties and applications. Whether you’re interested in understanding the role of coordination complexes in biological systems or developing new materials for catalysis or drug delivery, coordination chemistry offers endless possibilities.
Throughout this article, we have uncovered nine astounding facts about coordination chemistry. From the discovery of new coordination compounds to the development of coordination polymers, this field continues to push the boundaries of scientific knowledge.
So, whether you’re a chemistry enthusiast or a student embarking on a journey into the world of coordination chemistry, remember that there is always more to learn and discover. Embrace the complexity and beauty of coordination chemistry, and let it inspire you to explore the wonders of the molecular world.
FAQs
Q: What is coordination chemistry?
A: Coordination chemistry is a branch of chemistry that studies the interactions between metal ions and ligands, which are molecules or ions capable of donating electron pairs to the metal.
Q: What are coordination compounds?
A: Coordination compounds, also known as coordination complexes, are compounds in which a central metal ion is surrounded by ligands bonded to it through coordination bonds.
Q: What are ligands?
A: Ligands are molecules or ions that form coordinate bonds with a metal ion by donating electron pairs. They can be either neutral or negatively charged.
Q: What are some applications of coordination chemistry?
A: Coordination chemistry has numerous applications, including catalysis, drug delivery, materials science, and bioinorganic chemistry.
Q: What is the importance of coordination polymers?
A: Coordination polymers are structures formed by linking metal ions or clusters with organic ligands. They have a wide range of applications, including gas storage, sensing, and molecular recognition.
Q: How does coordination chemistry contribute to medicine?
A: Coordination compounds are used in medicine for their ability to selectively bind to biological targets, making them valuable in the development of drug molecules.
Q: What is chelation therapy?
A: Chelation therapy is a medical treatment that involves the administration of a chelating agent to remove heavy metals from the body. The chelating agent forms stable complexes with the metal ions, facilitating their excretion.
Q: How does coordination chemistry contribute to environmental remediation?
A: Coordination compounds can be used in environmental remediation to sequester and remove toxic metal ions from polluted sites.
Q: What are some future directions in coordination chemistry research?
A: Current research in coordination chemistry is focused on developing new ligands, expanding the scope of coordination chemistry to non-traditional metals, and exploring new applications in areas such as sustainable energy and nanotechnology.
Coordination chemistry's captivating world doesn't end here! Keep exploring this fascinating field by learning about the intriguing properties of Lewis bases. These unique compounds play a crucial role in forming complex structures, much like ligands in coordination compounds. So, if you're eager to expand your knowledge and unravel more chemical mysteries, dive into our next article and prepare to be amazed!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
