Complex ions, also known as coordination complexes, are fascinating structures that play a crucial role in the field of chemistry. These compounds are formed when a central metal atom or ion is surrounded by a coordinated array of ligands. While complex ions are widely studied, there are several surprising facts about them that many people may not be aware of. From their diverse colors to their unique magnetic properties, complex ions continue to captivate scientists and enthusiasts alike. In this article, we will explore 16 surprising facts about complex ions that will broaden your understanding of these intricate structures. So, let’s dive in and unravel the secrets of complex ions!
Key Takeaways:
- Complex ions are formed by bonding a central metal ion with surrounding molecules or ions, creating vibrant colors and playing crucial roles in biological systems and industry applications.
- Transition metals, ligands, and coordination numbers all contribute to the diverse properties and behaviors of complex ions, making them essential in redox reactions, chelation therapy, and catalytic activity.
Complex ions are formed by the bonding of a central metal ion with surrounding molecules or ions.
Complex ions, also known as coordination complexes, involve the donation of electron pairs from the surrounding molecules or ions to the central metal ion, forming a coordinated structure.
Transition metals are commonly found in complex ions.
Transition metals, such as iron, copper, and cobalt, are often found at the center of complex ions due to their ability to form multiple oxidation states and their capacity to bond with various ligands.
Ligands play a crucial role in the formation of complex ions.
Ligands are molecules or ions that donate electron pairs to the central metal ion, creating coordination bonds. They can be neutral molecules or negatively charged ions.
Complex ions can have different coordination numbers.
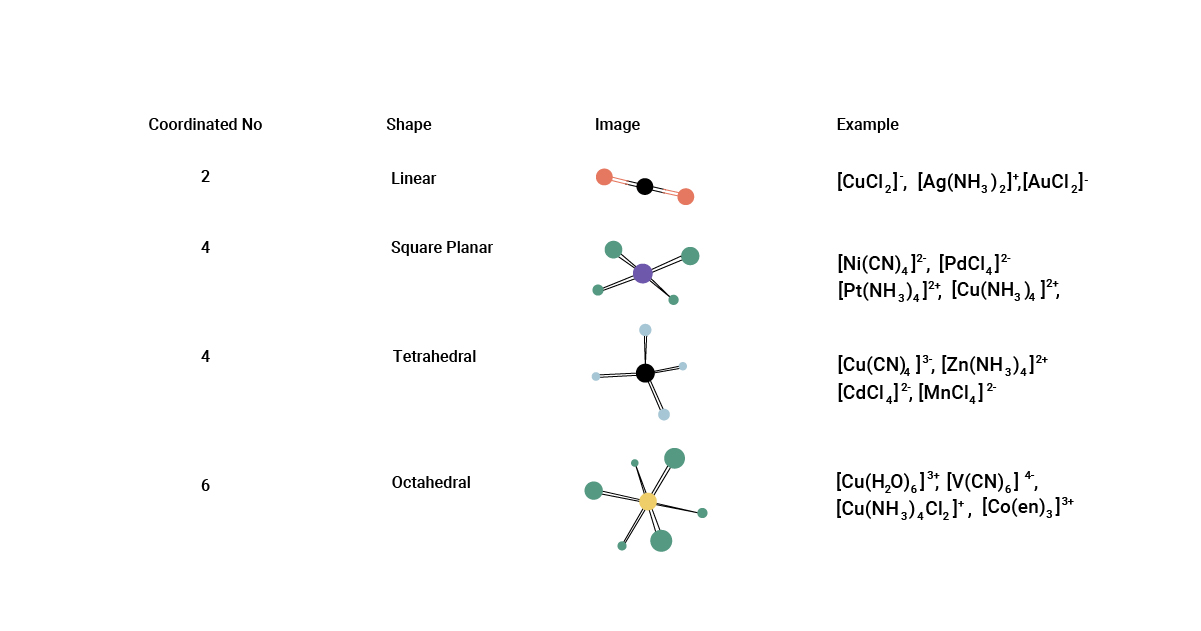
The coordination number refers to the number of ligands directly bonded to the central metal ion. It can range from 2 to 12 or even higher, depending on the size and charge of the metal ion.
The color of complex ions can vary.
Complex ions often exhibit vibrant colors due to the interaction of light with the d-orbitals of the central metal ion. The color of the complex depends on the nature of the ligands and the metal ion involved.
Complex ions can exhibit paramagnetism or diamagnetism.
Paramagnetic complex ions have unpaired electrons and are attracted to a magnetic field, while diamagnetic complex ions have all their electrons paired and are repelled by a magnetic field.
Complex ions play a crucial role in biological systems.
In living organisms, certain metal ions form complex ions with biomolecules such as proteins and enzymes to facilitate important biochemical processes, including oxygen transport and electron transfer.
Complex ions are widely used in industry.
Complex ions find applications in various industrial processes, including catalysis, electroplating, and extraction of metals from ores. They can also be used as dyes and pigments.
Isomerism is possible in complex ions.
Complex ions can exhibit different structural isomers, where the connectivity of ligands around the central metal ion varies, resulting in distinct physical and chemical properties.
Complex ions can undergo redox reactions.
Complex ions can undergo oxidation-reduction reactions, where the central metal ion changes its oxidation state. This makes them essential in many biological and industrial redox processes.
Chelation therapy involves the use of complex ions.
Chelation therapy utilizes complex ions to treat heavy metal poisoning by forming stable complexes with toxic metal ions, allowing them to be safely eliminated from the body.
Complex ions have diverse geometries.
Depending on the coordination number and the nature of ligands, complex ions can adopt various geometries, including octahedral, tetrahedral, square planar, and trigonal bipyramidal.
Complex ions can be optically active.
If a complex ion contains chiral ligands or a chiral central metal ion, it can exhibit optical activity, rotating the plane of polarized light passing through it.
Complex ions can form stable complexes with Lewis acids and bases.
In addition to ligands, complex ions can also interact with Lewis acids or bases, forming stable coordination compounds that have distinct chemical and physical properties.
Complex ions can act as catalysts.
Certain complex ions, particularly those containing transition metal ions, can exhibit catalytic activity, facilitating chemical reactions by providing an alternate reaction pathway with lower activation energy.
Complex ions can exhibit pH-dependent behavior.
The behavior of complex ions can vary with changes in pH. The protonation or deprotonation of ligands can alter their ability to coordinate with the central metal ion, affecting the overall stability of the complex.
Conclusion
The world of complex ions is fascinating and full of surprises. From their intricate structures to their unique properties, these compounds continue to captivate chemists and researchers alike. Whether it’s their ability to form stable complexes or their involvement in important chemical reactions, complex ions play a crucial role in various fields, including medicine, environmental science, and materials chemistry.Exploring the 16 surprising facts about complex ions has shed light on their significance and versatility. Understanding how they can change the color of a solution, influence the behavior of catalysts, or even impact the efficacy of certain drugs opens up new possibilities for scientific advancements.As we continue to delve deeper into the mysteries of complex ions, it is evident that there is still much to learn and discover. With ongoing research and technological advancements, we can expect to uncover even more surprising facts about these fascinating compounds and their potential applications.
FAQs
Q: What are complex ions?
A: Complex ions are charged species comprising a central metal ion surrounded by ligands. These ligands are typically coordination compounds or molecules that can donate electrons to form coordinate bonds with the metal ion.
Q: What is the significance of complex ions?
A: Complex ions have various practical applications. They play a crucial role in catalysis, biological processes, environmental remediation, and materials science. They also serve as coordination complexes in many metalloprotein structures.
Q: How do complex ions form?
A: Complex ions form when ligands bind to a central metal ion, resulting in the formation of coordination bonds. The coordination bonds create a three-dimensional structure around the metal ion, giving rise to complex ions.
Q: How do complex ions affect color?
A: Complex ions can absorb certain wavelengths of light, leading to the appearance of color in solutions. The electronic transitions within the complex ion’s structure determine the color observed.
Q: Can complex ions be toxic?
A: Some complex ions can be toxic, depending on the specific metal and ligands involved. For example, certain heavy metal complex ions can pose environmental and health hazards if not properly handled or disposed of.
Exploring complex ions is just the beginning of your journey into the fascinating world of chemistry. Dive deeper into coordination chemistry to uncover its astounding facts and principles. Ligands, essential components of complex ions, hold captivating secrets waiting to be discovered. Crystal field theory provides an unbelievable framework for understanding the behavior of these ions in various environments. Continue your exploration and prepare to be amazed by the wonders of chemistry.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
