When it comes to understanding the intricate nature of chemical bonding and how it influences the properties of transition metal complexes, Ligand Field Theory is an essential concept to grasp. This theory delves into the interaction between the metal ion and the surrounding ligands, providing insights into the colors, magnetic properties, and reactivity of these compounds.
In this article, we will explore 16 fascinating facts about Ligand Field Theory that will deepen your understanding of this crucial branch of chemistry. From the origins of the theory to its applications in various fields, we will delve into the enigmatic world of Ligand Field Theory and unravel the mysteries behind its principles.
Key Takeaways:
- Ligand Field Theory helps us understand why some metal compounds have vibrant colors, and it’s crucial for designing catalysts and predicting the stability of metal complexes. It’s like a secret code that unlocks the mysteries of transition metal behavior!
- By considering the influence of ligands on metal d orbitals, Ligand Field Theory explains the magnetic, electronic, and geometric properties of transition metal complexes. It’s like a special pair of glasses that reveals the hidden world of coordination compounds!
Ligand Field Theory provides insights into the color of transition metal compounds.
By considering the splitting of d orbitals in the presence of ligands, Ligand Field Theory can explain why certain compounds appear colored.
It was first proposed by Hans Bethe and John Hasbrouck van Vleck.
In the late 1920s, Bethe and van Vleck independently developed the theoretical framework for Ligand Field Theory.
Ligand Field Theory helps explain the magnetic properties of transition metal complexes.
By considering the spin and orbital contributions to the magnetic moment, Ligand Field Theory can account for the observed magnetic behavior of these complexes.
The Ligand Field Theory is based on the Crystal Field Theory.
Crystal Field Theory provides a simplified model for Ligand Field Theory by considering only the electrostatic interaction between the metal center and the ligands.
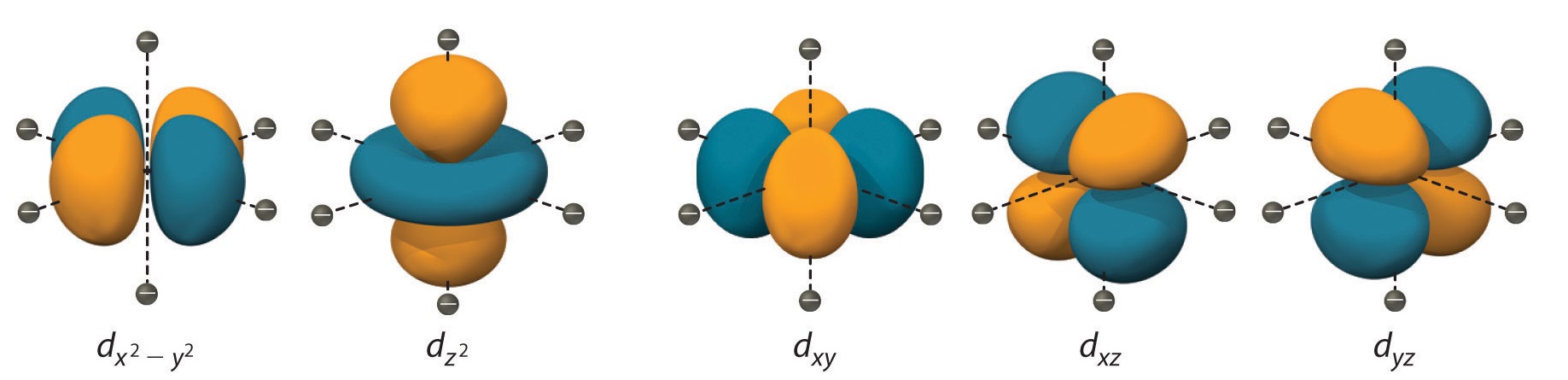
It considers the influence of ligands on the energy of d orbitals.
Ligand Field Theory explains how ligands can either raise or lower the energy of the metal center’s d orbitals, leading to different electronic configurations and properties.
Ligand Field Theory is crucial in understanding the stability of transition metal complexes.
By analyzing the strength of ligand-metal interactions, Ligand Field Theory helps predict the stability of complexes and their reactivity.
It can explain the phenomenon of Jahn-Teller distortion.
Ligand Field Theory provides insights into why certain transition metal complexes undergo geometric distortions to achieve lower energy states, known as Jahn-Teller distortions.
Ligand Field Theory helps in the design of catalysts.
Understanding the electronic structure of transition metal complexes through Ligand Field Theory is critical in designing efficient catalysts for various chemical reactions.
It explains the bonding and electronic structure in coordination compounds.
Ligand Field Theory provides a framework for understanding the nature of bonding and the electronic structure in coordination compounds.
Ligand Field Theory considers the different shapes of ligands.
The shape and size of ligands in a complex can influence the splitting of d orbitals, leading to variations in electronic configurations and properties.
It helps explain the phenomenon of ligand substitution in transition metal complexes.
Ligand Field Theory can predict and rationalize the substitution of ligands in transition metal complexes based on the electronic and steric effects.
Ligand Field Theory is used in spectroscopic techniques.
By considering the energy differences between d orbitals, Ligand Field Theory helps interpret spectroscopic data, such as UV-Vis and EPR spectra.
It considers both sigma and pi bonding in transition metal-ligand interactions.
Ligand Field Theory recognizes that both sigma and pi bonding play a role in the interaction between transition metal centers and ligands.
Ligand Field Theory can explain the thermodynamic stability of metal complexes.
By analyzing the relative energies of different electronic configurations, Ligand Field Theory contributes to understanding the thermodynamic stability of metal complexes.
It helps explain the reactivity of transition metal complexes in chemical reactions.
Ligand Field Theory sheds light on the influence of ligands on the rate and mechanism of reactions involving transition metal complexes.
Ligand Field Theory is a cornerstone of modern inorganic chemistry.
With its ability to explain and predict the properties and behavior of transition metal complexes, Ligand Field Theory has become a fundamental tool in the field of inorganic chemistry.
These 16 enigmatic facts about Ligand Field Theory highlight its significance and broad applications in understanding the behavior of transition metal complexes. By incorporating Ligand Field Theory into our understanding of inorganic chemistry, we gain valuable insights into the fascinating world of coordination compounds.
Conclusion
Ligand field theory is a fascinating topic in the field of chemistry that helps us understand the behavior of transition metal complexes. Through this theory, we have discovered a multitude of enigmatic facts that shed light on the intricate interactions between ligands and metal ions.
From the understanding of ligand field theory, we now comprehend why certain complexes exhibit unique colors, why they have different magnetic properties, and how the electronic structure of the metal ion influences their reactivity. This theory has revolutionized our comprehension of complex systems and has immense applications in catalysis, drug design, and materials science.
Exploring the enigmatic facts about ligand field theory has opened up new avenues for scientific inquiry and has expanded our knowledge of chemical bonding and molecular behavior. As researchers continue to delve deeper into this field, we can expect even more exciting discoveries that will further advance our understanding of the complex world of chemistry.
FAQs
Q: What is ligand field theory?
A: Ligand field theory is a concept in chemistry that explains the behavior of transition metal complexes by considering the interactions between the metal ion and the ligands surrounding it.
Q: How does ligand field theory help in understanding complex systems?
A: Ligand field theory provides insights into why certain complexes exhibit unique colors, magnetic properties, and reactivity, allowing us to better understand and manipulate complex chemical systems.
Q: What are the applications of ligand field theory?
A: Ligand field theory has wide-ranging applications in catalysis, drug design, and materials science, playing a crucial role in the development of new drugs, catalysts, and advanced materials.
Q: How does ligand field theory impact catalysis?
A: Ligand field theory helps in designing and understanding catalytic systems by providing insights into the coordination environment of metal ions and their influence on reaction mechanisms.
Q: Are there any ongoing research efforts in the field of ligand field theory?
A: Yes, researchers continue to explore ligand field theory to uncover new insights into complex systems, with an aim to develop innovative applications and further expand our understanding of chemical bonding and molecular behavior.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
