Phase diagrams are a crucial tool in understanding the behavior of substances under different conditions of temperature and pressure. These diagrams visually represent the relationship between these variables and the phases of a substance, such as solid, liquid, and gas. They provide valuable insights into the physical and chemical properties of materials, enabling scientists and engineers to make informed decisions in various fields, including chemistry, materials science, and engineering.
In this article, we dive into the fascinating world of phase diagrams and uncover 18 astounding facts that will expand your knowledge on this essential topic. From the discovery of phase diagrams to their practical applications, we will explore the secrets and intricacies hiding within these charts. So, buckle up and prepare to be amazed as we take a journey through the wonders of phase diagrams.
Key Takeaways:
- Phase diagrams show how substances change with temperature and pressure, helping scientists understand when things melt, vaporize, or turn into gas without becoming liquid.
- Phase diagrams are like treasure maps for scientists, guiding them to discover new materials and understand how different phases of matter behave under specific conditions.
What is a Phase Diagram?
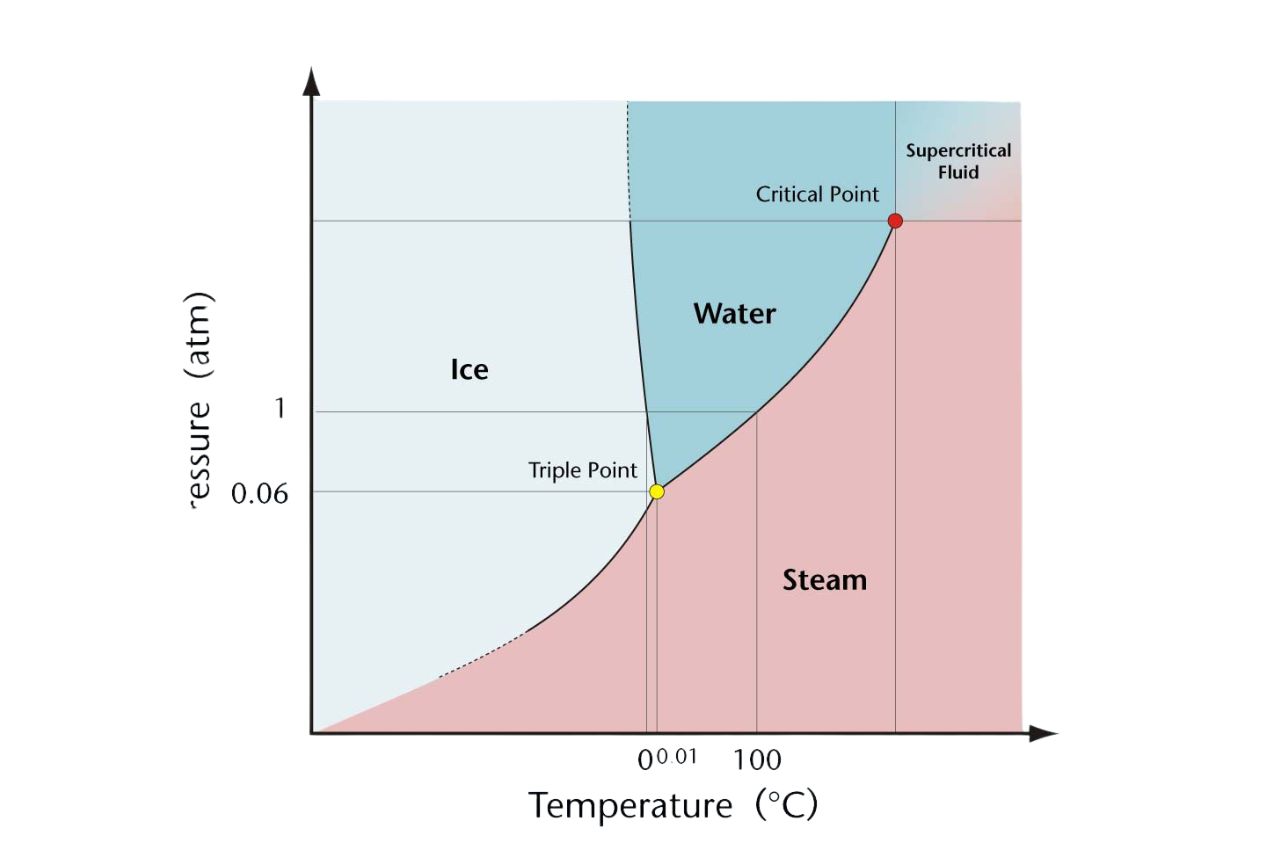
A phase diagram is a graphical representation of the different phases of a substance as it undergoes changes in temperature and pressure.
Three Key Phases
The three primary phases depicted in a phase diagram are solid, liquid, and gas.
Phase Transitions
Phase diagrams provide information about the conditions under which phase transitions occur, such as melting, vaporization, and sublimation.
Critical Point
Each substance has a critical point on the phase diagram, representing the temperature and pressure at which the distinction between liquid and gas phases disappears.
Triple Point
The triple point on a phase diagram is the unique set of conditions where all three phases of a substance coexist in equilibrium.
Sublimation
Some substances can undergo sublimation, transitioning directly from the solid phase to the gas phase without passing through the liquid phase.
Liquid-Gas Equilibrium
The line separating the liquid and gas phases on a phase diagram is known as the vapor-liquid equilibrium line.
Phase Diagrams for Mixtures
Phase diagrams can also be created for mixtures of substances, representing the changes in phase as the composition of the mixture is varied.
Eutectic Point
In a binary phase diagram, the eutectic point represents the lowest temperature and composition at which a mixture of two substances can solidify.
Phase Diagrams in Material Science
Phase diagrams are widely used in material science to study the behavior and properties of materials at different temperatures and pressures.
Pressure Effects
The phase diagram of a substance can be affected by changes in pressure, leading to shifts in the boundaries between different phases.
Understanding Phase Equilibrium
Phase diagrams provide a visual representation of phase equilibrium, helping scientists understand how different phases coexist under specific conditions.
Energy Changes
Phase transitions are associated with specific energy changes, such as heat absorption or release, which are crucial for various industrial and scientific applications.
Phase Diagrams in Discovering New Materials
Scientists use phase diagrams to explore and discover new materials with unique properties by identifying regions of stability for specific phases.
Liquid Crystal Phase Diagrams
Phase diagrams are extensively used in the study of liquid crystals, a distinct state of matter that exhibits properties of both liquids and solids.
High-Pressure Phase Diagrams
Phase diagrams at high pressures reveal the behavior of substances in extreme conditions, providing valuable insights into the physics and chemistry of matter.
Effect of Impurities
Impurities in a substance can significantly affect its phase diagram, altering the boundaries between phases and influencing the overall behavior of the material.
Phase Diagrams in Industrial Applications
Phase diagrams play a crucial role in various industrial processes, such as determining the optimal conditions for materials synthesis, casting, and heat treatment.
Conclusion
In conclusion, phase diagrams are a crucial tool in understanding the behavior of substances under different conditions. They provide valuable information about the various phases of matter and the changes they undergo.By analyzing phase diagrams, scientists and engineers can determine the appropriate conditions required to achieve a specific phase or phase transition. They are commonly used in various fields, including chemistry, materials science, and engineering.Understanding phase diagrams allows us to predict the behavior of substances when subjected to different temperatures and pressures. This knowledge is vital for a range of applications, including the design of new materials, the optimization of manufacturing processes, and the development of innovative technologies.Overall, phase diagrams are an essential tool in the study and application of chemistry. They offer valuable insights into the behavior of substances and provide a foundation for further research and technological advancements.
FAQs
Q: What is a phase diagram?
A: A phase diagram is a graphical representation that shows the different phases of a substance at various combinations of temperature and pressure.
Q: Why are phase diagrams important?
A: Phase diagrams help us understand how substances behave under different conditions, which is crucial for a variety of applications, including materials science and engineering.
Q: What are the different phases of matter?
A: The different phases of matter include solid, liquid, and gas. Depending on the substance and the conditions, additional phases such as plasma and supercritical fluid may also exist.
Q: How do phase diagrams help in materials science?
A: Phase diagrams help materials scientists understand how various materials undergo phase transitions, enabling them to create new materials with specific properties and functionalities.
Q: Can phase diagrams predict the behavior of all substances?
A: Phase diagrams are limited to substances that exhibit well-defined phase changes under different temperature and pressure conditions. They may not be applicable to all substances, especially those with complex molecular structures.
Q: How are phase diagrams constructed?
A: Phase diagrams are constructed based on empirical data obtained through experiments and observations. Thermodynamic principles and mathematical models are used to interpret the data and create the graphical representation.
Intrigued by phase diagrams' fascinating facts? Delve deeper into their significance with our article on 11 intriguing facts about phase diagrams. Uncover more secrets behind these powerful tools used in chemistry, materials science, and beyond. Explore how phase diagrams help scientists understand the behavior of substances under various conditions and their crucial role in developing new materials. Gain a comprehensive understanding of phase diagrams and their applications in our ever-evolving world of science and technology. Don't miss out on this opportunity to expand your knowledge and appreciate the beauty of phase diagrams!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
