The eutectic point is a fascinating concept in the field of chemistry that often leaves people baffled. It refers to the specific temperature at which a eutectic mixture of two or more substances completely melts or solidifies, without any change in composition. This phenomenon is something truly remarkable and offers valuable insights into the behavior of compounds and their interactions.
In this article, we will explore 13 unbelievable facts about the eutectic point that will surely ignite your curiosity. From its profound applications in various industries to its role in metallurgy, we will uncover the hidden secrets behind this extraordinary chemical occurrence. So get ready to delve into the fascinating world of the eutectic point and discover how it shapes the world around us.
Key Takeaways:
- The eutectic point is a critical temperature in chemistry that influences the properties of materials, from alloys to chocolate, and even pharmaceuticals, making it a key concept in various industries.
- Understanding the eutectic point helps scientists and engineers create stronger materials, develop better welding techniques, and even improve the taste and texture of chocolate, showing its wide-ranging impact in the real world.
What is the Eutectic Point?
The eutectic point is a critical concept in chemistry that refers to the specific temperature at which a eutectic mixture undergoes a phase change.
How is the Eutectic Point Determined?
The eutectic point is determined through careful experimentation and observation of the phase behavior of different mixtures. Scientists use a variety of techniques, such as differential scanning calorimetry (DSC) and thermal analysis, to identify this critical temperature.
The Significance of the Eutectic Point
The eutectic point is of great importance in various industries, particularly in metallurgy and materials science. It allows engineers and researchers to optimize the properties of alloys, ensuring desirable characteristics such as improved strength, resistance to corrosion, and enhanced durability.
Eutectic Point and Freezing Points
The eutectic point is often associated with the freezing of substances. When a eutectic mixture is cooled below its eutectic temperature, it solidifies into a homogeneous solid, rather than forming separate crystals. This unique behavior is crucial in the manufacturing of numerous materials.
Microstructure Formation at the Eutectic Point
The eutectic point plays a key role in the formation of specific microstructures in materials. For example, in eutectic alloys, the solidified microstructure consists of alternating layers or lamellae of different phases, resulting in enhanced mechanical properties.
Eutectic Point in Pharmaceutical Industry
The pharmaceutical industry utilizes the concept of the eutectic point in drug formulation. Eutectic mixtures can improve the solubility and bioavailability of poorly soluble drugs, enhancing their therapeutic efficacy.
Examples of Eutectic Mixtures
There are numerous examples of eutectic mixtures, including salt and ice (used in ice cream-making), tin-silver solder, and eutectic alloys such as bronze and certain types of steel.
Eutectic Point in Food Science
The eutectic point has applications in the field of food science, particularly in the production of chocolate. By controlling the eutectic point, manufacturers can ensure the desired texture and consistency of chocolate products.
Supercooling and the Eutectic Point
Supercooling occurs when a liquid is cooled below its freezing point without solidifying. The eutectic point can influence the extent of supercooling and the subsequent solidification behavior of the substance.
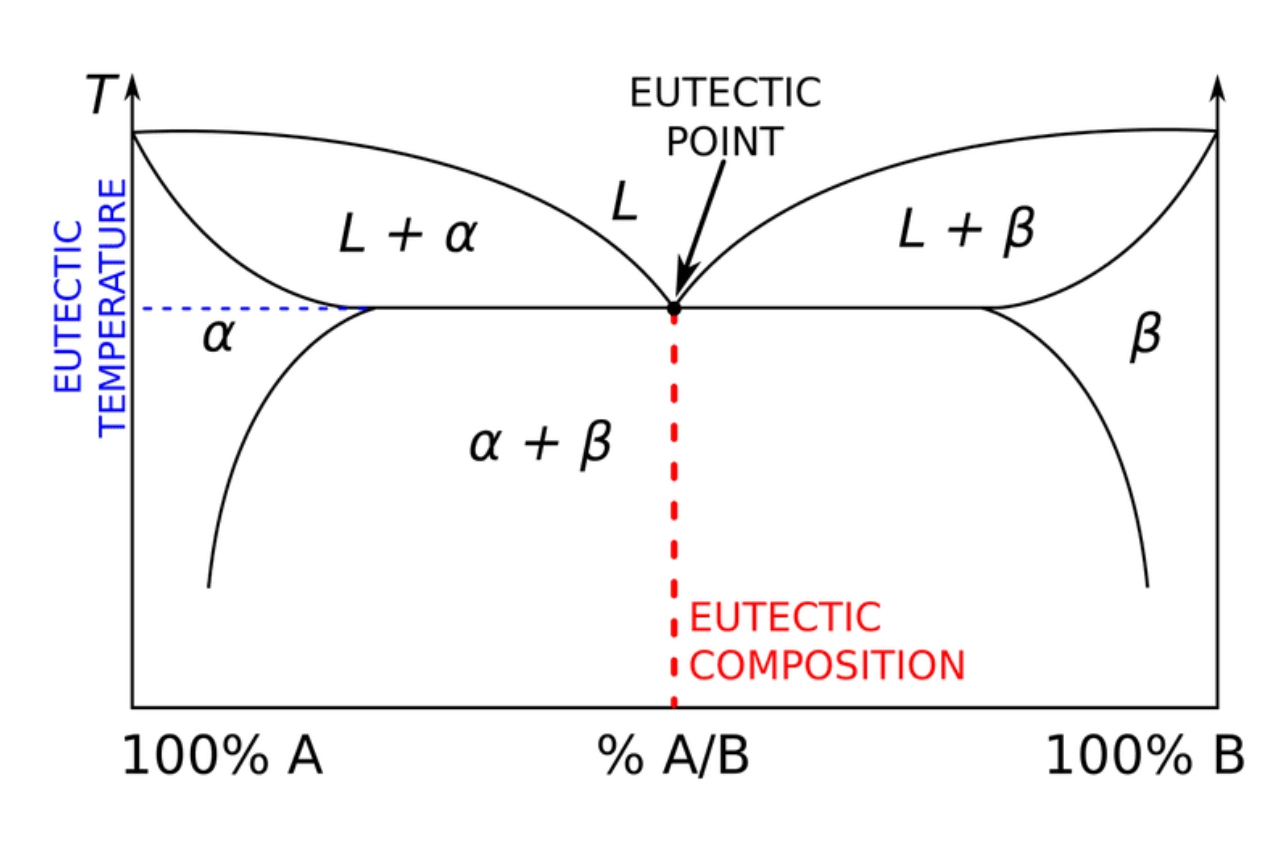
Binary Phase Diagrams and the Eutectic Point
The eutectic point is graphically represented in binary phase diagrams, which illustrate the relationship between temperature, composition, and phase. These diagrams provide valuable insights into the behavior of eutectic systems.
Eutectic Point and Industrial Applications
The eutectic point is a crucial parameter in various industrial applications, such as the production of specialized alloys, ceramics, and semiconductors. Understanding the eutectic point allows engineers to develop materials with tailored properties.
Eutectic Point and Welding
In welding, the eutectic point is of significance as it determines the behavior of the molten metal during solidification. This knowledge helps welding professionals create strong and defect-free joints.
Advancements in Eutectic Point Research
Ongoing research is focused on further understanding the eutectic point and developing new applications. Scientists are exploring novel materials and mixtures where the eutectic point plays a critical role.
Conclusion
The eutectic point is a fascinating concept in chemistry that has many surprising facts associated with it. Understanding the eutectic point can provide valuable insights into the behavior of chemical compounds and their mixtures.
From its historical significance to its practical applications in various industries, the eutectic point serves as a crucial point of reference for scientists and engineers alike. It plays a vital role in fields such as metallurgy, pharmaceuticals, and materials science.
By knowing the eutectic point, researchers can determine the optimal conditions for mixing substances, ensuring the desired properties and characteristics are achieved. This knowledge is utilized in the development of new materials with improved performance and enhanced functionality.
Exploring the 13 unbelievable facts about the eutectic point has not only expanded our understanding of this phenomenon but also showcased the wonders and intricacies of the chemical world around us.
In conclusion, the eutectic point serves as a crucial foundation for countless applications, shaping new scientific discoveries and technological advancements.
FAQs
Q: What is the eutectic point?
A: The eutectic point is the specific composition at which a mixture of two or more substances has the lowest melting point.
Q: Why is the eutectic point important?
A: The eutectic point provides crucial information about the behavior and properties of chemical compounds when mixed together. It helps in determining the optimal conditions for achieving specific characteristics and desired outcomes.
Q: How is the eutectic point determined?
A: The eutectic point is determined through experimentation, by gradually varying the proportions of the substances in the mixture and observing the changes in the melting point. The point at which the melting point reaches its minimum is identified as the eutectic point.
Q: What are some practical applications of the eutectic point?
A: The knowledge of the eutectic point is utilized in various industries such as metallurgy, pharmaceuticals, and materials science. It helps in the development of alloys, the formulation of drugs, and the production of materials with specific properties.
Q: Can the eutectic point be different for different substances?
A: Yes, the eutectic point varies depending on the specific substances involved in the mixture. Each compound or combination has its own unique eutectic point.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
