Autoprotolysis is a fascinating phenomenon that occurs in chemistry, revealing the inherent complexity and interconnectedness of the molecular world. It refers to the self-ionization of a substance, where molecules of the same substance transfer protons to one another, resulting in the formation of ions. This process occurs in various substances, such as water, where water molecules can act as both an acid and a base, donating and accepting protons.
In this article, we will delve into the extraordinary facts about autoprotolysis that will not only broaden your understanding of this intriguing chemical phenomenon but also highlight its significance in many scientific disciplines. From the role of autoprotolysis in maintaining the pH balance in our bodies to its applications in industrial processes, get ready to discover the hidden depths of autoprotolysis and appreciate its impact on the world around us.
Key Takeaways:
- Autoprotolysis is a unique chemical process where solvents like water can act as both acids and bases, influencing pH and playing a crucial role in biological systems.
- Understanding autoprotolysis helps predict the strength of acids and bases, and its ongoing research opens up possibilities for new discoveries in chemistry.
Autoprotolysis is a fascinating chemical phenomenon.
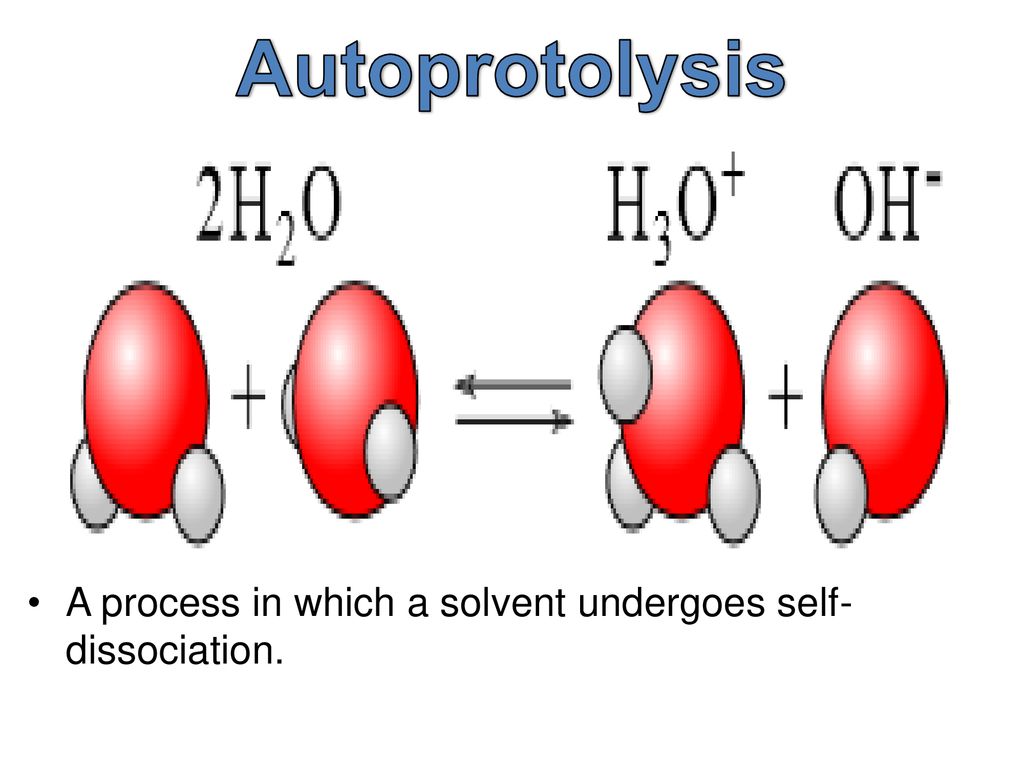
Autoprotolysis refers to the self-ionization process of a solvent, where molecules of the solvent act as both acids and bases to form ions. This unique property allows for the spontaneous transfer of protons without the presence of an external acid or base.
Water is the most well-known example of autoprotolysis.
One of the most common examples of autoprotolysis is the self-ionization of water. In this process, two water molecules react with each other, forming hydronium ions (H3O+) and hydroxide ions (OH-). This equilibrium reaction plays a crucial role in the pH scale and many chemical reactions.
Autoprotolysis occurs in various solvents.
While water is the most widely studied solvent for autoprotolysis, this phenomenon can occur in other solvents as well. Ammonia, for example, exhibits self-ionization, forming ammonium ions (NH4+) and amide ions (NH2-).
Autoprotolysis affects the acidity and basicity of a solvent.
The autoprotolysis of a solvent influences its acid and base strengths. The presence of equal concentrations of hydronium and hydroxide ions in water, for instance, results in a neutral pH of Solvents with a higher concentration of hydronium ions are considered acidic, while those with a higher concentration of hydroxide ions are considered basic.
The autoprotolysis constant quantifies the extent of self-ionization.
In autoprotolysis, the equilibrium constant, known as the autoprotolysis constant (Kw), is used to measure the extent of self-ionization. For water, Kw is equal to 1.0 x 10^-14 at 25°C, indicating that water is slightly acidic due to the presence of hydronium and hydroxide ions.
Autoprotolysis plays a crucial role in acid-base chemistry.
Autoprotolysis forms the foundation of acid-base chemistry and contributes to various chemical reactions. Understanding the self-ionization process helps in determining the strength of acids and bases, as well as predicting the outcomes of acid-base reactions.
Autoprotolysis allows for the concept of amphoteric substances.
Amphoteric substances are compounds capable of acting as both acids and bases. Autoprotolysis enables certain chemicals, such as aluminum hydroxide, to exhibit amphoteric properties, reacting as an acid with bases and as a base with acids.
The autoprotolysis of water affects its physical properties.
The self-ionization of water influences its physical properties, including boiling point, freezing point, and density. These changes arise from the altered concentration of hydronium and hydroxide ions and their effects on intermolecular forces.
Autoprotolysis is temperature dependent.
The rate of autoprotolysis increases with an increase in temperature. Higher temperatures provide more thermal energy, leading to greater molecular collisions and increased self-ionization of the solvent.
Autoprotolysis is reversible.
Just like any equilibrium reaction, autoprotolysis is reversible. This means that the formation of hydronium and hydroxide ions can revert back to water molecules under certain conditions, maintaining a dynamic equilibrium.
Autoprotolysis has implications in biological systems.
The autoprotolysis of water holds great significance in biological systems. It impacts the pH balance within living organisms and influences enzymatic reactions, cellular processes, and overall bodily functions.
Autoprotolysis is a subject of ongoing research.
Despite being a well-established phenomenon, autoprotolysis continues to be an active area of research. Scientists explore new solvents, study the effects of temperature and pressure, and investigate the implications of autoprotolysis in various fields of chemistry.
Conclusion
In conclusion, autoprotolysis is a fascinating phenomenon that occurs in certain compounds, particularly in solvents such as water. These 12 extraordinary facts about autoprotolysis shed light on the intricate nature of chemical reactions and the role of proton transfer in establishing equilibrium. From the discovery of autoprotolysis to its applications in various fields, such as catalysis and acid-base chemistry, autoprotolysis continues to captivate scientists and researchers worldwide.
FAQs
Q: What is autoprotolysis?
A: Autoprotolysis is a self-ionization process that occurs within certain compounds, where one molecule donates a proton (H+) to another molecule, resulting in the formation of a conjugate acid and a conjugate base.
Q: What are some examples of compounds that undergo autoprotolysis?
A: The most common example of autoprotolysis is the self-ionization of water, where water molecules transfer protons to form hydronium (H3O+) and hydroxide (OH-) ions. Other examples include base-catalyzed protic solvents like alcohols and carboxylic acids.
Q: How does autoprotolysis affect the acidity and basicity of a compound?
A: Autoprotolysis plays a crucial role in establishing the acid-base equilibrium of a compound. It determines the concentration of H+ and OH- ions, which in turn affects the pH and acidity/basicity of the solution or compound.
Q: What are some applications of autoprotolysis?
A: Autoprotolysis has various applications in fields such as catalysis, acid-base chemistry, and electrochemistry. It helps in understanding the behavior of solvents, determining acid-base strengths, and developing efficient chemical reactions.
Q: Can autoprotolysis occur in non-aqueous solvents?
A: Yes, autoprotolysis can occur in non-aqueous solvents as well. For example, in protic solvents like alcohols and carboxylic acids, the transfer of protons between molecules can lead to autoprotolysis.
Q: Is autoprotolysis reversible?
A: Yes, autoprotolysis is a reversible process. The transfer of protons can occur in both directions, which helps maintain equilibrium between the formation of conjugate acid and conjugate base.
Q: How does temperature affect autoprotolysis?
A: Temperature has a significant impact on autoprotolysis. As temperature increases, the rate of proton transfer also increases, leading to a higher concentration of H+ and OH- ions. Additionally, the equilibrium constant of autoprotolysis is temperature-dependent.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
