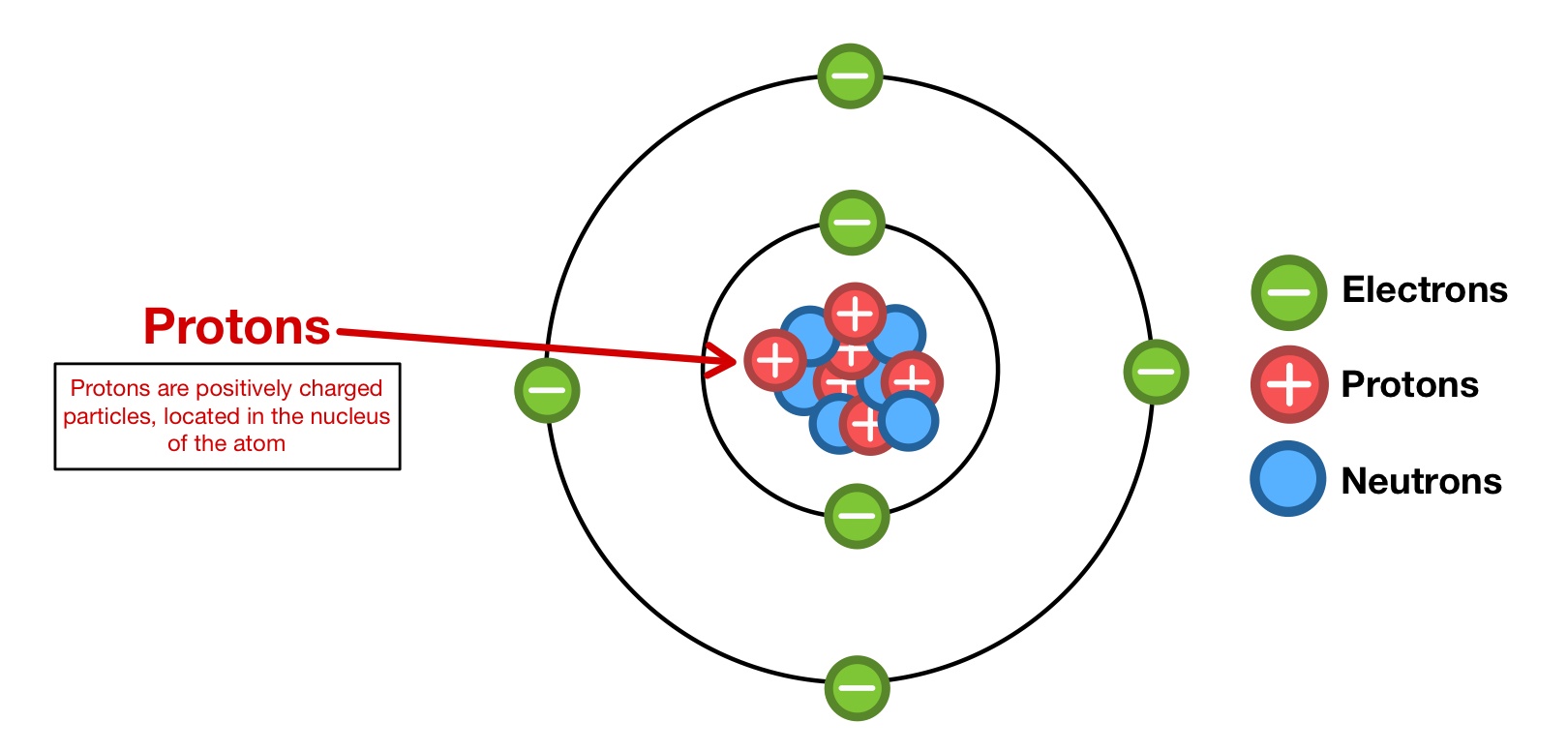
Protons, one of the fundamental particles of matter, are intriguing and fascinating in many ways. These positively charged subatomic particles are found in the nucleus of atoms, along with neutrons. While most people may be familiar with the concept of protons from their high school chemistry classes, there is so much more to discover about these tiny particles.
In this article, we will delve deeper into the world of protons and uncover 9 mind-blowing facts that will leave you in awe of their significance in the field of chemistry. From their crucial role in atomic structure to their contribution to the formation of chemical bonds, protons have a profound impact on our understanding of the world around us. So, get ready to expand your knowledge and explore the wonders of protons!
Key Takeaways:
- Protons are essential building blocks of matter, determining an atom’s identity and playing a crucial role in the overall charge of an atom. They are stable, have mass, and power the stars through fusion.
- Protons are not just tiny particles in atoms; they are used in cutting-edge cancer treatments and particle accelerators for studying high-energy physics. Their behavior is governed by quantum mechanics, making them even more fascinating!
A Fundamental Building Block of Matter
Proton is one of the three fundamental particles that make up an atom, alongside neutrons and electrons. It carries a positive electrical charge and is found in the nucleus of an atom.
The Positive Charge Carrier
As protons have a positive charge, they play a crucial role in determining the overall charge of an atom. The number of protons in an atom defines its atomic number, which determines the element’s identity.
Mass of Protons
Protons have a mass of approximately 1.67 x 10^-27 kilograms, which is roughly 1,836 times the mass of an electron.
Stable in the Nucleus
Protons are inherently stable within the atomic nucleus, meaning they do not decay over time.
Proton-Proton Fusion Powers the Stars
In the core of stars, protons undergo nuclear fusion, combining to form helium nuclei and releasing enormous amounts of energy in the process.
Subatomic Particle Accelerators
Protons are widely used in particle accelerators to study the fundamental nature of matter, high-energy physics, and medical treatments.
Proton Therapy for Cancer Treatment
Proton therapy is a cutting-edge cancer treatment that uses high-energy protons to target and destroy tumors, minimizing damage to surrounding healthy tissues.
Spin and Magnetic Moment
Protons possess an intrinsic property called spin, which gives rise to their magnetic moment. This property plays a crucial role in interactions with magnetic fields.
Quantum Mechanics and Proton Wave Function
Protons, like other subatomic particles, are encompassed by the principles of quantum mechanics. Their behavior is described by a wave function, which captures their probabilistic nature.
Conclusion
The proton is a fascinating subatomic particle that plays a crucial role in the world of chemistry. From its discovery to its essential function in atomic nuclei, exploring the properties and behavior of protons has expanded our understanding of the universe. Its ability to interact with other particles and its role in chemical reactions make it a fundamental building block for all matter.
Knowing these mind-blowing facts about protons not only deepens our appreciation for the complexity of the atomic world but also highlights the interconnectedness of all aspects of science. The study of protons continues to unveil new insights and applications in various fields, including chemistry, physics, and even medicine.
The more we delve into the mysteries of protons, the more we realize how they shape the world around us. As scientific knowledge advances, our understanding of protons will undoubtedly continue to evolve, unlocking even more astonishing discoveries.
FAQs
1. What is a proton?
A proton is a subatomic particle that carries a positive electric charge and is found in the nucleus of an atom.
2. What is the mass of a proton?
The mass of a proton is approximately 1.67 x 10^-27 kilograms.
3. How was the proton discovered?
The proton was discovered by Ernest Rutherford in 1919 through his famous gold foil experiment.
4. What is the charge of a proton?
A proton carries a positive electric charge, which is equal in magnitude but opposite in sign to the negative charge of an electron.
5. What role do protons play in chemical reactions?
Protons play a vital role in chemical reactions by determining the chemical properties of elements and facilitating bond formation.
6. Can protons be found outside of the nucleus?
No, protons are typically confined within the nucleus of an atom and are not found outside in normal circumstances.
7. Can protons change into other particles?
Protons can be converted into neutrons or other particles through processes such as beta decay or nuclear reactions.
8. Are protons involved in generating electricity?
Yes, protons are involved in generating electricity through the movement of electrons in a conductor.
9. Can protons be manipulated or controlled?
Yes, protons can be manipulated and controlled through technologies such as particle accelerators and nuclear reactors.
Protons' fascinating properties make them essential to our universe. Delving deeper into protons reveals even more captivating facts, like how ProtonMail harnesses their power for secure communication or the way proton pump inhibitors treat stomach acid issues. Exploring proton motive force also uncovers intriguing insights into cellular energy production. Each topic offers a unique perspective on these incredible subatomic particles, showcasing their versatility and importance in various fields.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
