Chemistry enthusiasts and learners, get ready to have your minds blown! In the fascinating world of chemistry, the concept of basicity constants of weak bases is a topic that deserves our attention. Understanding the basicity constants of weak bases is crucial for predicting their behavior in various chemical reactions and reactions with acids.
But what exactly are basicity constants? In simple terms, they are quantitative measures of the strength of weak bases. These constants help chemists determine the extent to which a base can donate electrons and accept protons.
In this article, we will explore 12 mind-blowing facts about basicity constants of weak bases. Prepare to delve into the world of chemical equilibria, pKb values, and pH calculations. From the influence of molecular structure to the effects of temperature, each fact will unveil intriguing aspects of basicity constants and expand our knowledge of the fascinating realm of chemistry.
Key Takeaways:
- Basicity constants measure the strength of weak bases and help predict their reactivity, stability, and pH of a solution. They play a crucial role in understanding chemical reactions and drug discovery.
- Basicity constants are not fixed values and can change with temperature and concentration. They provide insights into the properties of weak bases and are fundamental in acid-base titrations.
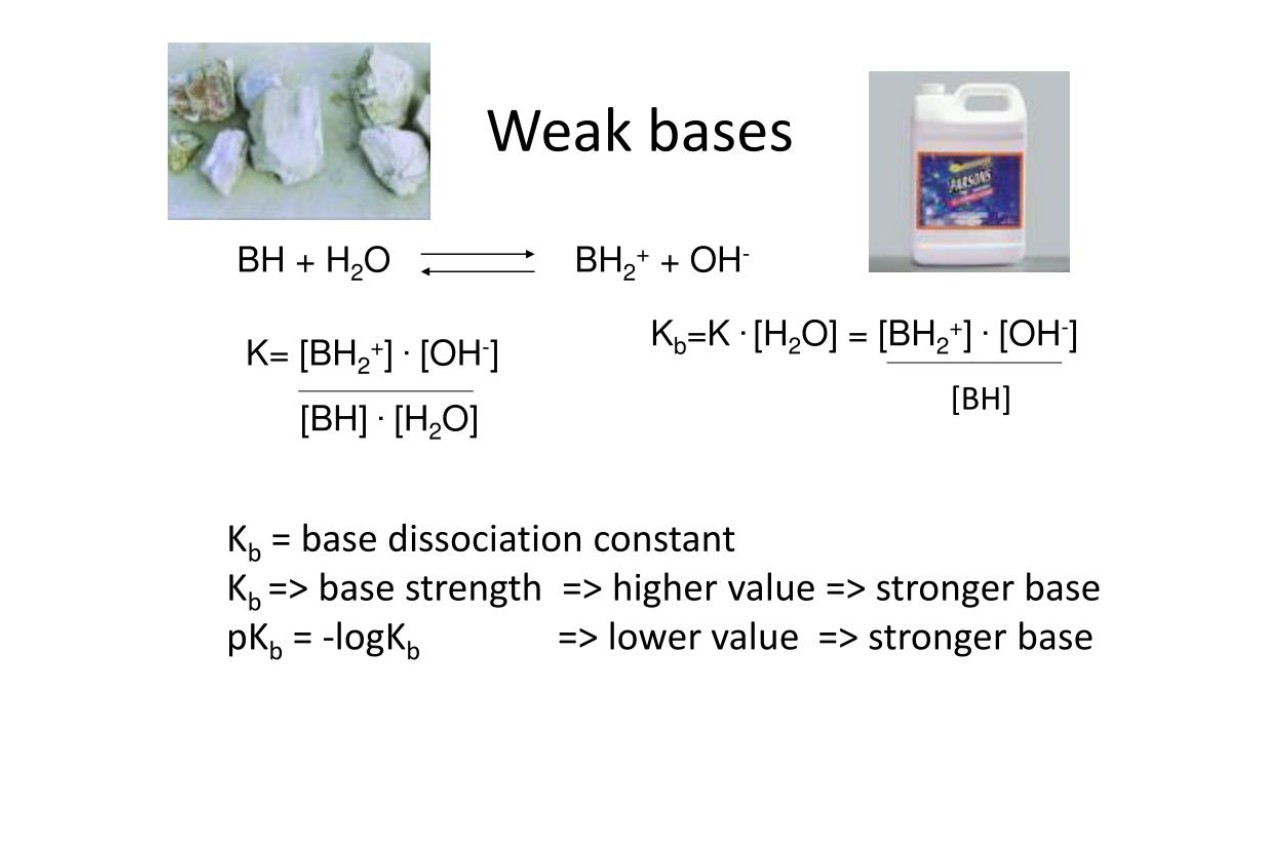
The basicity constant measures the strength of a weak base.
The basicity constant is a numerical value that quantifies the strength of a weak base. It provides important information about the ability of a substance to accept protons and form conjugate acids.
Basicity constants vary depending on the solution’s temperature and concentration.
The basicity constant of a weak base is not a fixed value and can change with varying conditions. Factors such as temperature and concentration of the solution can influence the strength of the base.
The basicity constant reflects the stability of the base’s conjugate acid.
A higher basicity constant indicates a more stable conjugate acid, meaning the weak base has a greater ability to accept protons and form a strong acid.
Acidity and basicity constants are related.
Acidity constants and basicity constants are connected through the concept of equilibrium. High basicity constants correspond to low acidity constants and vice versa.
Basicity constants help predict the reactivity of weak bases.
By analyzing the value of the basicity constant, chemists can anticipate the reactivity of a weak base. A higher constant typically indicates greater reactivity.
Dissociation constant and basicity constant are distinct.
The dissociation constant measures the degree of dissociation of a weak base in solution, while the basicity constant specifically focuses on the strength of the base in accepting protons.
The pKA value is related to the basicity constant.
The pKA value, which is the negative logarithm of the acidity constant, is inversely related to the basicity constant. Higher pKA values correspond to lower basicity constants.
Basicity constants help determine the pH of a solution.
Knowing the basicity constant of a weak base allows chemists to calculate the pH of a solution by analyzing the concentration of the base and its conjugate acid.
Basicity constants provide insights into chemical reactions.
Understanding the basicity constants of weak bases is crucial in predicting how they will react with other chemical compounds. This knowledge assists in the design and synthesis of new molecules.
Basicity constants are utilized in drug discovery.
By studying the basicity constants of various chemical compounds, scientists can identify potential drug candidates and evaluate their efficacy and safety profiles.
The concept of basicity constants extends beyond aqueous solutions.
While basicity constants are commonly discussed in relation to aqueous solutions, they are applicable to other solvents as well. Chemists consider the solvent’s nature and properties when studying basicity constants.
Basicity constants are fundamental to acid-base titrations.
Basicity constants play a crucial role in acid-base titrations, as they help determine the volume and concentration of a reactant needed to reach a neutralization point.
These 12 mind-blowing facts about basicity constants of weak bases reveal the intricate nature of this area of study in chemistry. Understanding the basicity constants provides crucial insights into the reactivity, stability, and properties of weak bases, allowing scientists to make advancements in various fields such as drug discovery and chemical synthesis. So, next time you encounter basicity constants, remember the fascinating facts that lie behind these seemingly simple numerical values.
Conclusion
In conclusion, the basicity constants of weak bases are a fascinating aspect of chemistry. Understanding these constants can provide valuable insights into the strength and behavior of these bases. We have explored 12 mind-blowing facts about basicity constants, delving into topics such as their significance in acid-base equilibria, the factors influencing their values, and their practical applications in various fields.
From the understanding of pKa values to the determination of dissociation constants, these facts shed light on the complex world of weak bases and their basicity constants. By studying these constants, chemists can gain a deeper understanding of the properties and reactivity of different compounds, leading to advancements in fields such as pharmaceuticals, materials science, and environmental chemistry.
The study of basicity constants is ever-evolving, with new research uncovering exciting discoveries and applications. It is an area of chemistry that continues to push the boundaries of our knowledge and enhance our understanding of the fundamental principles that govern chemical reactions.
FAQs
Q: What are basicity constants?
Basicity constants are quantitative measures of the strength of weak bases. They represent the tendency of a base to accept a proton and form a conjugate acid.
Q: How are basicity constants determined?
Basicity constants can be determined through various experimental methods, such as measuring the pH change in a solution upon addition of the base or using spectroscopic techniques to monitor the extent of protonation.
Q: What factors influence the values of basicity constants?
The values of basicity constants are influenced by factors such as the electronic structure of the base, steric hindrance around the basic site, and the solvent medium in which the reaction occurs.
Q: How are basicity constants used in chemistry?
Basicity constants play a crucial role in acid-base equilibria calculations, designing and optimizing chemical reactions, understanding the reactivity of compounds, and predicting the behavior of weak bases in various chemical processes.
Q: Can basicity constants be used to compare the strength of different bases?
Yes, basicity constants provide a comparative measure of the strength of weak bases. Higher values indicate stronger bases, while lower values indicate weaker bases.
Unraveling basicity constants' intricacies is just the beginning of your chemistry journey. Dive deeper into the fascinating world of <chemical equilibrium>, where reactions reach a delicate balance. Explore the unique properties of <aqueous solutions>, nature's ultimate solvent. Lastly, grasp the fundamentals of <acid-base reactions> through the lens of Bronsted-Lowry theory. Each topic offers a captivating glimpse into the complex tapestry of chemical phenomena, waiting for curious minds like yours to uncover their secrets.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
