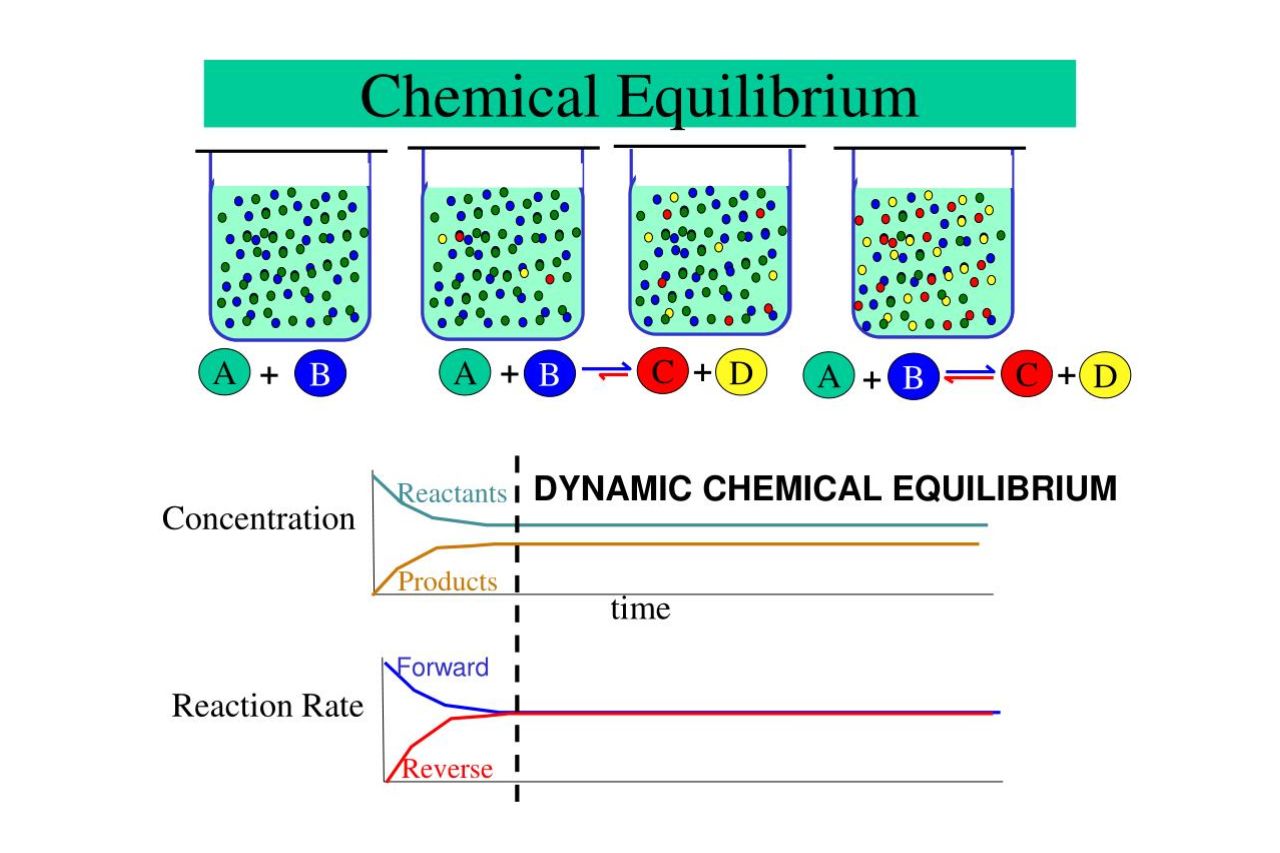
Chemical equilibrium is a fascinating concept that plays a crucial role in understanding the behavior of chemical reactions. It occurs when the forward and reverse reactions in a system reach a point where the concentrations of reactants and products remain constant over time. The study of chemical equilibrium has profound implications in various fields, including chemistry, biology, and environmental science.
In this article, we will delve into the captivating world of chemical equilibrium and explore ten intriguing facts that will expand your knowledge and appreciation of this fundamental concept. From the principles established by renowned chemists to real-life applications, these facts will shed light on the complex and dynamic nature of chemical equilibrium.
Key Takeaways:
- Chemical equilibrium is a delicate balance where reactions reach a state of balance. It’s like a seesaw where the forward and reverse reactions are equal, keeping everything in harmony.
- Chemical equilibrium is like a dynamic dance party where molecules are constantly switching partners between reactants and products. It’s a balanced, ever-changing process crucial for many chemical reactions in nature and our daily lives.
Chemical Equilibrium: A Delicate Balance
Chemical equilibrium is a fascinating concept that governs the behavior of reactions. It occurs when the rates of the forward and reverse reactions are equal, resulting in a state of balance. This delicate balance is crucial for many chemical processes to occur in nature and in our daily lives.
The Dynamic Nature of Chemical Equilibrium
Contrary to popular belief, chemical equilibrium is not a static state. Instead, it is a dynamic process where molecules are constantly interconverting between reactants and products. This dynamic nature allows for the continuous formation and decomposition of compounds at a balanced rate.
Le Chatelier’s Principle: Shifting the Balance
Le Chatelier’s Principle is a fundamental concept related to chemical equilibrium. It states that when a system at equilibrium is subjected to a change in temperature, pressure, or concentration, the system will shift to counteract that change. This principle explains how the equilibrium position can be influenced and manipulated.
The Role of Catalysts
Catalysts play a crucial role in chemical equilibrium. They are substances that increase the rate of both the forward and reverse reactions, allowing equilibrium to be reached faster. However, catalysts do not affect the position of the equilibrium; they only expedite the attainment of equilibrium.
Equilibrium Constant: The Key to Quantifying Equilibrium
The equilibrium constant (K) is a numerical value that represents the position of the equilibrium. It is calculated by expressing the concentrations of the products and reactants at equilibrium. The value of K provides insights into the relative quantities of reactants and products in a chemical system.
Shifts in Equilibrium: The Effect of Changing Conditions
Various factors can cause a shift in the equilibrium position. Changes in temperature, pressure, or concentration can alter the balance between reactants and products. Understanding these shifts is essential in predicting and manipulating chemical reactions for industrial and environmental applications.
Physical and Chemical Equilibrium
Chemical equilibrium is not limited to reactions involving only chemicals. It also extends to physical processes such as the evaporation of liquids or the dissolution of solids. These physical equilibria involve the balance between molecules in different phases and their respective rates of conversion.
Equilibrium in Biological Systems
Chemical equilibrium is not exclusive to the realm of chemistry laboratories. It also plays a vital role in biological systems such as the human body. Many biochemical processes, including enzyme-catalyzed reactions and cellular respiration, rely on the delicate balance of chemical equilibrium.
Reversible Reactions and Equilibrium
Chemical equilibrium is closely associated with reversible reactions. Reversible reactions can proceed in both the forward and reverse directions, depending on the conditions. When a reversible reaction reaches equilibrium, the concentrations of reactants and products become constant, indicating a stable state.
The Equilibrium of Solubility
Equilibrium is also observed in solubility processes. The solubility of a substance in a solvent depends on various factors such as temperature and pressure. At equilibrium, the rate of dissolution is equal to the rate of crystallization, resulting in a saturated solution.
Chemical equilibrium is a captivating phenomenon that governs the behavior of reactions and systems in the world of chemistry. Understanding its principles and applications is crucial in various fields, from industrial processes to biological systems. Explore the intricacies of chemical equilibrium and discover the remarkable balance that exists in the world of molecules.
Conclusion
Chemical equilibrium is a fascinating concept that governs a wide range of chemical reactions. Understanding the principles of chemical equilibrium is crucial for scientists and chemists alike. In this article, we have explored 10 captivating facts about chemical equilibrium.We learned that chemical equilibrium occurs when the rates of the forward and backward reactions are equal, resulting in a constant concentration of reactants and products. This dynamic balance allows for the formation of different chemical species.Moreover, we discovered that chemical equilibrium is influenced by factors such as temperature, pressure, and concentration. These factors can shift the equilibrium position, driving the reaction in one direction or another.Additionally, we explored Le Chatelier’s principle, which states that a system at equilibrium will adjust to counteract any changes imposed upon it. Understanding this principle enables chemists to predict how factors such as temperature or pressure will impact the equilibrium.Chemical equilibrium plays a crucial role in various fields, including industrial processes, environmental studies, and biochemistry. It’s a fundamental concept that underpins the understanding of chemical systems and reactions.In conclusion, chemical equilibrium is a complex and fascinating phenomenon that scientists continue to study and explore. Its principles offer insights into how reactions proceed and how to manipulate them for practical applications. As we delve deeper into the world of chemistry, understanding chemical equilibrium becomes increasingly vital.
FAQs
1. What is chemical equilibrium?
Chemical equilibrium is a state in which the rates of the forward and backward reactions in a chemical system are equal, resulting in a constant concentration of reactants and products.
2. What factors can influence chemical equilibrium?
Factors such as temperature, pressure, and concentration can influence chemical equilibrium. Changes in these factors can shift the equilibrium position and alter the concentrations of reactants and products.
3. What is Le Chatelier’s principle?
Le Chatelier’s principle states that a system at equilibrium will adjust to counteract any changes imposed upon it. For example, if the concentration of a reactant is increased, the system will shift to consume the excess reactant and establish a new equilibrium.
4. How is chemical equilibrium relevant in industrial processes?
Chemical equilibrium is critical in industrial processes, such as the production of ammonia and the Haber-Bosch process. Understanding and manipulating equilibrium conditions allow for the efficient production of desired products.
5. How does chemical equilibrium relate to environmental studies?
Chemical equilibrium is relevant in environmental studies, especially in understanding the equilibrium between different forms of pollutants in air, water, and soil. It helps in determining the fate and transport of contaminants in the environment.
6. How is chemical equilibrium significant in biochemistry?
Chemical equilibrium is essential in biochemistry as it governs many biological processes, such as enzyme-catalyzed reactions and the binding of ligands to proteins. Understanding equilibrium is crucial for understanding biological systems and their functions.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
