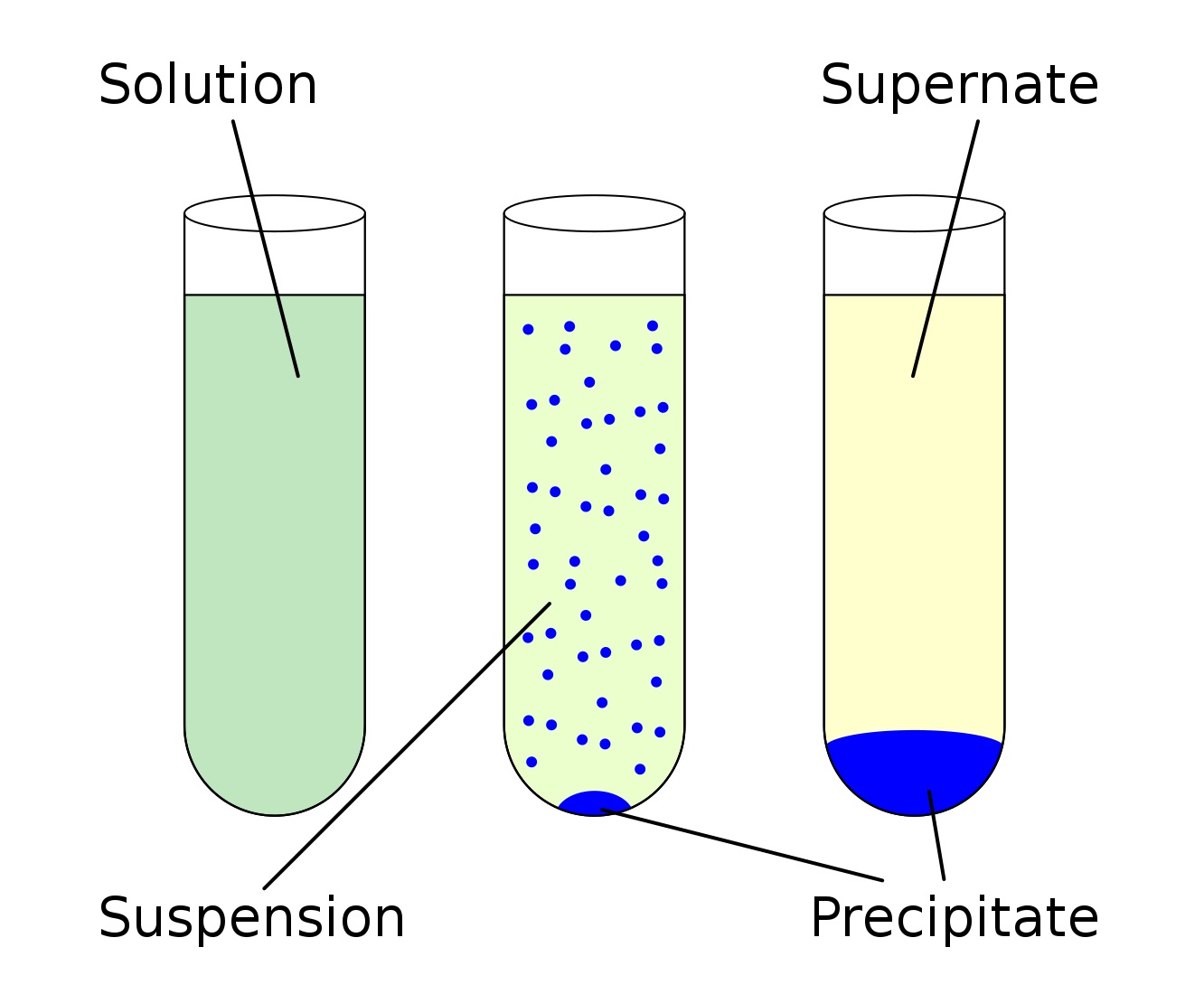
Solubility product is a fundamental concept in chemistry that plays a crucial role in understanding the behavior of chemicals in solution. It is a measure of the extent to which a substance can dissolve in a solvent, and it is represented by the equilibrium constant for the dissolution reaction. The solubility product constant, usually denoted as Ksp, provides valuable information about the solubility of a compound and the formation of precipitates.
In this article, we will delve into the intriguing world of solubility product and explore 18 captivating facts that will broaden your understanding of this concept. From the factors affecting solubility to the significance of Ksp in various chemical reactions, you are about to embark on a fascinating journey through the complexities of solubility chemistry. So, fasten your seatbelts and get ready to unravel the mysteries of solubility product!
Key Takeaways:
- Solubility product determines if a compound will dissolve or form a solid. Factors like temperature, pH, and common ions affect it, and it’s crucial for predicting precipitation reactions.
- Understanding solubility product helps predict when insoluble salts will form and how temperature and common ions impact solubility. It’s essential for various fields of chemistry and real-world applications.
The Concept of Solubility Product
Solubility product is a fundamental concept in chemistry that relates to the equilibrium between a solid solute and its ions in a solution. It is denoted by the symbol Ksp and represents the product of the concentrations of the ions raised to the power of their stoichiometric coefficients in a balanced chemical equation. This concept plays a crucial role in understanding the dissolution and precipitation of different compounds.
The Formula for Solubility Product
The general formula for solubility product is represented as Ksp = [A+]^m[B-]^n, where A+ and B- are the ions of the solute and m, n are their stoichiometric coefficients, respectively. This formula helps calculate the solubility of a compound under specific conditions and provides insight into the behavior of solutions.
Solubility Product and Precipitation
Solubility product determines whether a compound will remain in solution or precipitate as a solid. When the ion product (Q) is greater than the solubility product (Ksp), precipitation occurs as the solution is saturated with the compound. Conversely, when Q is less than Ksp, the solution is unsaturated, and no precipitation takes place.
Factors Affecting Solubility Product
The solubility product of a compound can be influenced by various factors, such as temperature, pressure, and the presence of other substances in the solution. Changes in these factors can lead to changes in the solubility and, consequently, the value of the solubility product.
The Relationship Between Ksp and Solubility
The value of the solubility product, Ksp, is directly related to the solubility of the compound. Higher solubility corresponds to a larger Ksp value, indicating a greater concentration of the dissolved ions in the solution.
Common Ion Effect on Solubility Product
The presence of a common ion in a solution can have a significant impact on the solubility product. The addition of a common ion reduces the solubility of a compound, as it shifts the equilibrium towards the formation of the solid precipitate.
Solubility Product and pH
Changes in pH can affect the solubility of certain compounds by altering the concentration of ions in the solution. This can, in turn, influence the value of the solubility product.
Applications of Solubility Product
The concept of solubility product finds widespread applications in various fields of chemistry. It is used in analytical chemistry to determine the concentration of ions in a solution, in environmental chemistry to study the solubility of pollutants, and in pharmaceutical chemistry to understand the dissolution behavior of drugs.
Utilizing Solubility Product for Precipitation Reactions
Solubility product is essential in predicting and understanding precipitation reactions. By comparing Q to Ksp, it is possible to determine if a precipitate will form and the conditions under which this will occur.
Solubility Product and the Common Ion Effect
The common ion effect is an essential phenomenon related to solubility product. It describes the reduction in solubility of a compound when a solution containing a common ion is added, resulting in the shift of equilibrium towards precipitation.
The Role of Solubility Product in Solubility Rules
Solubility product plays a key role in determining solubility rules for various compounds. By comparing the value of Ksp with a given compound’s actual solubility, it is possible to predict whether the compound will dissolve or precipitate.
Solubility Product and the Dissolution of Ionic Compounds
Understanding solubility product is crucial in explaining the dissolution of ionic compounds. The balanced equation for the dissolution process can be derived from the solubility product expression, providing insights into the behavior of these compounds in solution.
Theoretical and Experimental Determination of Solubility Product
Solubility product values can be determined using various experimental techniques, such as measuring the concentration of ions in a saturated solution. These experimental values can then be compared to theoretical calculations based on equilibrium expressions to validate the solubility product concept.
The Effect of Temperature on Solubility Product
Temperature plays a significant role in determining the solubility product of a compound. In general, an increase in temperature leads to an increase in the solubility of most compounds, resulting in a higher Ksp value.
Solubility Product and Solubility Constant
The terms “solubility product” and “solubility constant” are often used interchangeably. Both terms refer to the mathematical representation of the equilibrium between a solute and its ions in a solution.
Solubility Product and the Stability of Complex Ions
Solubility product is also related to the stability of complex ions. Complex ions can form when ligands bind to metal ions, affecting their solubility and altering the value of the solubility product.
Solubility Product and the Formation of Insoluble Salts
Insoluble salts are formed when the solubility product of a compound is exceeded, leading to the precipitation of the insoluble solid. Solubility product plays a crucial role in understanding the conditions under which these salts form.
Using Solubility Product to Predict Precipitation Reactions
The solubility product can be used to predict whether or not a precipitation reaction will occur. By comparing the ion product (Q) to the solubility product (Ksp), it is possible to determine if a precipitate will form under given conditions.
Conclusion
In conclusion, understanding and exploring the concept of solubility product can greatly enhance our understanding of chemical reactions and the behavior of solutions. We have delved into 18 captivating facts about solubility product, covering various aspects such as the definition, factors affecting solubility, and applications in everyday life and industry.By studying the solubility product, we can predict whether a compound will dissolve in a given solvent and determine the concentration of ions in a saturated solution. We have also learned about the role of common-ion effect, temperature, and pressure in affecting solubility equilibrium.Additionally, we have explored fascinating applications of solubility product in fields such as pharmaceuticals, environmental science, and agriculture. From designing drug formulations to understanding the solubility of pollutants in water, solubility product plays a crucial role in various industries.Overall, the study of solubility product provides us with valuable insights into the behavior of solutions and helps us make informed decisions in various scientific and practical contexts.
FAQs
Q: What is solubility product?
A: Solubility product represents the mathematical product of the ion concentrations in a saturated solution of a compound at equilibrium.
Q: How is solubility product different from solubility?
A: Solubility refers to the maximum amount of solute that can dissolve in a given solvent, whereas solubility product is a specific numerical value calculated from the concentrations of ions in a saturated solution.
Q: What factors affect solubility product?
A: Factors such as temperature, pressure, presence of common ions, and the pH of the solution can affect the solubility product of a compound.
Q: How is solubility product used in pharmaceuticals?
A: Solubility product helps pharmaceutical scientists determine the optimal formulation of drugs, ensuring their effective delivery and absorption in the body.
Q: Can solubility product be used in environmental science?
A: Yes, solubility product is used to understand the solubility of pollutants in water and design effective strategies for their removal or remediation.
Q: Is solubility product only relevant in chemistry?
A: Solubility product has applications in various fields beyond chemistry, including environmental science, pharmaceuticals, agriculture, and materials science.
Solubility product plays a crucial role in understanding precipitation reactions and the formation of insoluble salts. Mastering this concept opens doors to predicting chemical behavior and applying it to real-world situations. Delving deeper into the intricacies of solubility product constants, such as Ksp, can further enhance your comprehension of this fascinating topic. By exploring the factors influencing solubility product and its relationship with other chemical principles, you'll gain a well-rounded understanding of this essential aspect of chemistry. Embark on a journey to unravel more captivating facts about solubility product and expand your knowledge in this field.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
