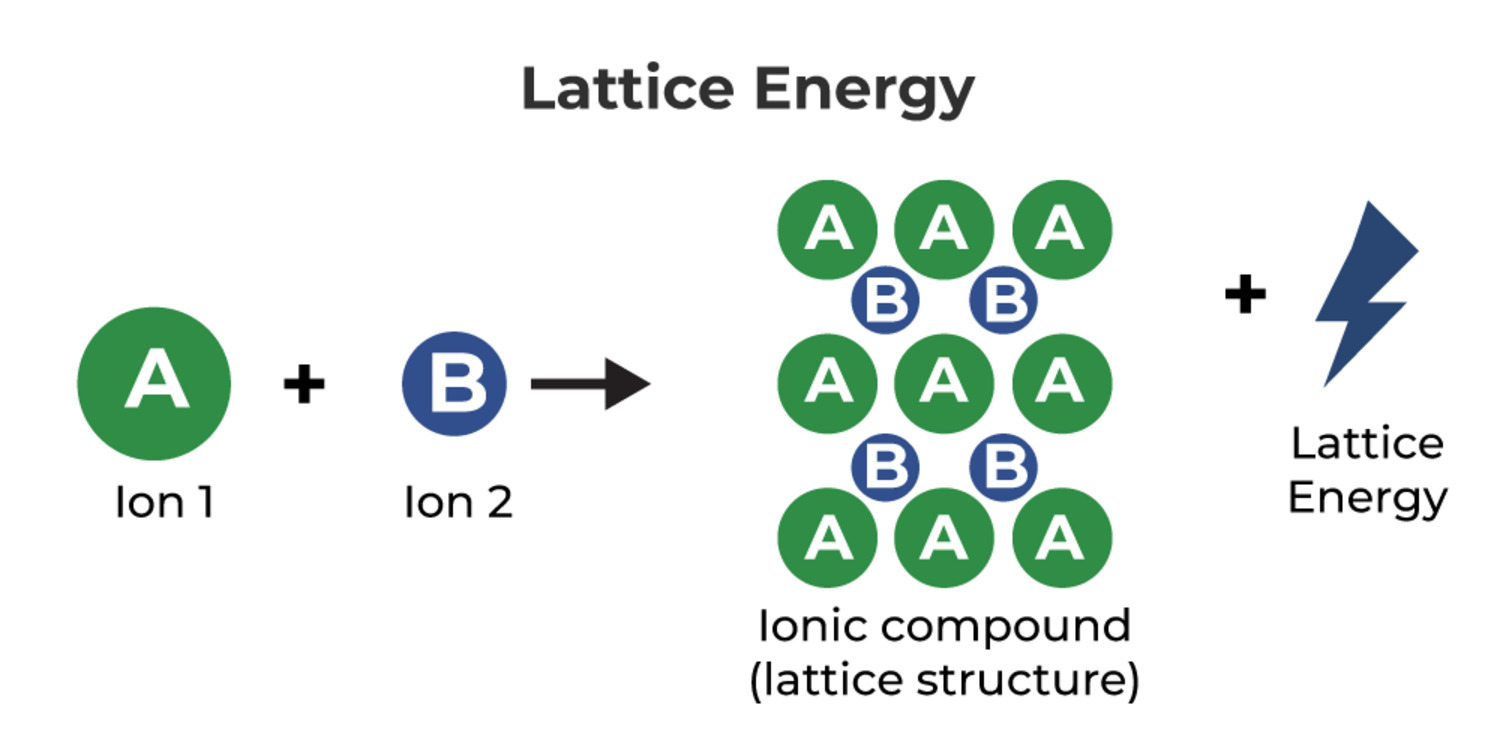
When it comes to understanding the properties and behavior of compounds, lattice energy plays a vital role in chemistry. Lattice energy is the energy released when ions come together to form a solid crystal lattice. This intriguing phenomenon is crucial in determining the stability and solubility of compounds, as well as their physical properties.
In this article, we will dive deep into the world of lattice energy and explore 12 surprising facts that will leave you fascinated. From its origin and factors affecting its magnitude, to its significance in different chemical processes, we will unravel the mysteries surrounding this essential concept in chemistry. So, buckle up and get ready to uncover some intriguing insights about lattice energy!
Key Takeaways:
- Lattice energy measures the strength of ionic bonds in compounds. Higher values mean stronger bonds and greater stability, affecting solubility, melting points, and even battery design.
- Ionic size, charges, and crystal arrangement influence lattice energy. It impacts chemical reactivity, crystal structure, and the enthalpy of formation, making it a crucial concept in chemistry.
Definition of Lattice Energy
Lattice energy is the energy released when gaseous ions combine to form a solid ionic compound. It is a measure of the strength of the ionic bonds in the crystal lattice structure.
Quantifying Lattice Energy
Lattice energy is typically expressed in units of kilojoules per mole (kJ/mol). Higher lattice energy values indicate stronger ionic bonds and greater stability of the compound.
Determining Factors
Lattice energy is influenced by various factors, including the charges on the ions, the sizes of the ions, and the arrangement of ions in the crystal lattice. These factors collectively determine the strength of the ionic bonds.
Relationship to Ionic Size
Larger ions have weaker attractions due to increased distance between the positive and negative charges. Therefore, compounds with larger ions tend to have lower lattice energy values.
Use in Solubility Predictions
Lattice energy is closely related to a compound’s solubility. Compounds with higher lattice energy values are typically less soluble in water, while those with lower lattice energy values are more likely to dissolve.
Influence on Melting and Boiling Points
Lattice energy is a significant factor in determining the melting and boiling points of ionic compounds. Higher lattice energy values generally translate to higher melting and boiling points.
Comparing Lattice Energy
When comparing compounds with the same ions, the lattice energy increases with increasing charges on the ions. For example, MgO has a higher lattice energy than NaCl due to the 2+ and 2- charges on the ions compared to the 1+ and 1- charges.
Role in Ionic Bond Strength
Lattice energy directly correlates with the strength of the ionic bonds in a compound. Compounds with higher lattice energy values have stronger ionic bonds, leading to more stable compounds.
Application in Batteries
The concept of lattice energy is crucial in understanding and designing battery systems. It influences the stability and reactivity of the compounds used in battery electrodes.
Impact on Crystal Structures
Lattice energy affects the crystal structure of ionic compounds. Compounds with higher lattice energy values tend to have more tightly packed crystal lattices.
Lattice Energy and Enthalpy of Formation
Lattice energy is a contributing factor to the enthalpy of formation, which measures the energy change when forming a compound from its elements. Higher lattice energy values increase the overall enthalpy of formation.
Influences on Chemical Reactivity
Lattice energy influences the chemical reactivity of compounds. Compounds with high lattice energy values are generally less reactive, while those with lower lattice energy values are more prone to chemical reactions.
These 12 surprising facts about lattice energy showcase its significance in understanding the stability, properties, and behavior of ionic compounds. Whether you’re a chemistry enthusiast or a student studying the subject, delving into the world of lattice energy will undoubtedly deepen your understanding of this fundamental concept.
So, let’s embrace the mysterious world of lattice energy and explore its intriguing role in the realm of chemistry!
Conclusion
In conclusion, lattice energy is a fascinating aspect of chemistry that plays a crucial role in determining the stability and properties of solid compounds. It is the energy released when gaseous ions come together to form a crystal lattice structure. Through various factors like ion size, charge, and arrangement, lattice energy can be altered and impact the overall characteristics of a compound.
In this article, we have explored 12 surprising facts about lattice energy. From its role in determining the solubility of salts to its relationship with the Born-Haber cycle, lattice energy is a concept that is integral to understanding the behavior of ionic substances. By understanding these facts, we gain a deeper insight into the fascinating world of chemical interactions at the molecular level.
FAQs
Q: What is lattice energy?
A: Lattice energy is the energy released when gaseous ions come together to form a crystal lattice structure.
Q: How is lattice energy measured?
A: Lattice energy is typically expressed in units of kilojoules per mole (kJ/mol).
Q: Does lattice energy affect the solubility of salts?
A: Yes, lattice energy plays a significant role in determining the solubility of salts. Higher lattice energy generally leads to lower solubility.
Q: What factors affect lattice energy?
A: Ion size, ion charge, and arrangement of ions within the crystal lattice are the key factors that affect lattice energy.
Q: Is lattice energy an intrinsic property of a compound?
A: Yes, lattice energy is a property that is specific to a particular compound and is determined by the types and arrangement of ions within its crystal lattice.
Q: How does lattice energy relate to the Born-Haber cycle?
A: Lattice energy is one of the components in the Born-Haber cycle, which is a series of hypothetical steps used to calculate the enthalpy change in forming an ionic compound from its constituent elements.
Q: Can lattice energy be negative?
A: No, lattice energy is always positive as it represents the energy released during the formation of a crystal lattice structure.
Q: Is there a correlation between lattice energy and melting point?
A: Generally, compounds with higher lattice energy tend to have higher melting points due to the stronger bonds between ions.
Q: Do all ionic compounds have lattice energy?
A: Yes, lattice energy is a characteristic of all ionic compounds as it is a measure of the energy required to break the crystal lattice structure.
Q: Can lattice energy be influenced by external factors?
A: Lattice energy is primarily determined by the characteristics of the ions involved. However, external factors such as pressure and temperature can influence lattice energy to some extent.
Exploring lattice energy's fascinating facts is just the beginning! Dive deeper into chemistry's captivating concepts with our articles on enthalpy's extraordinary impact, solid state chemistry's Schottky defect surprises, and crystal lattice's mesmerizing mysteries. Uncover more mind-blowing insights that'll leave you craving additional chemistry knowledge.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
