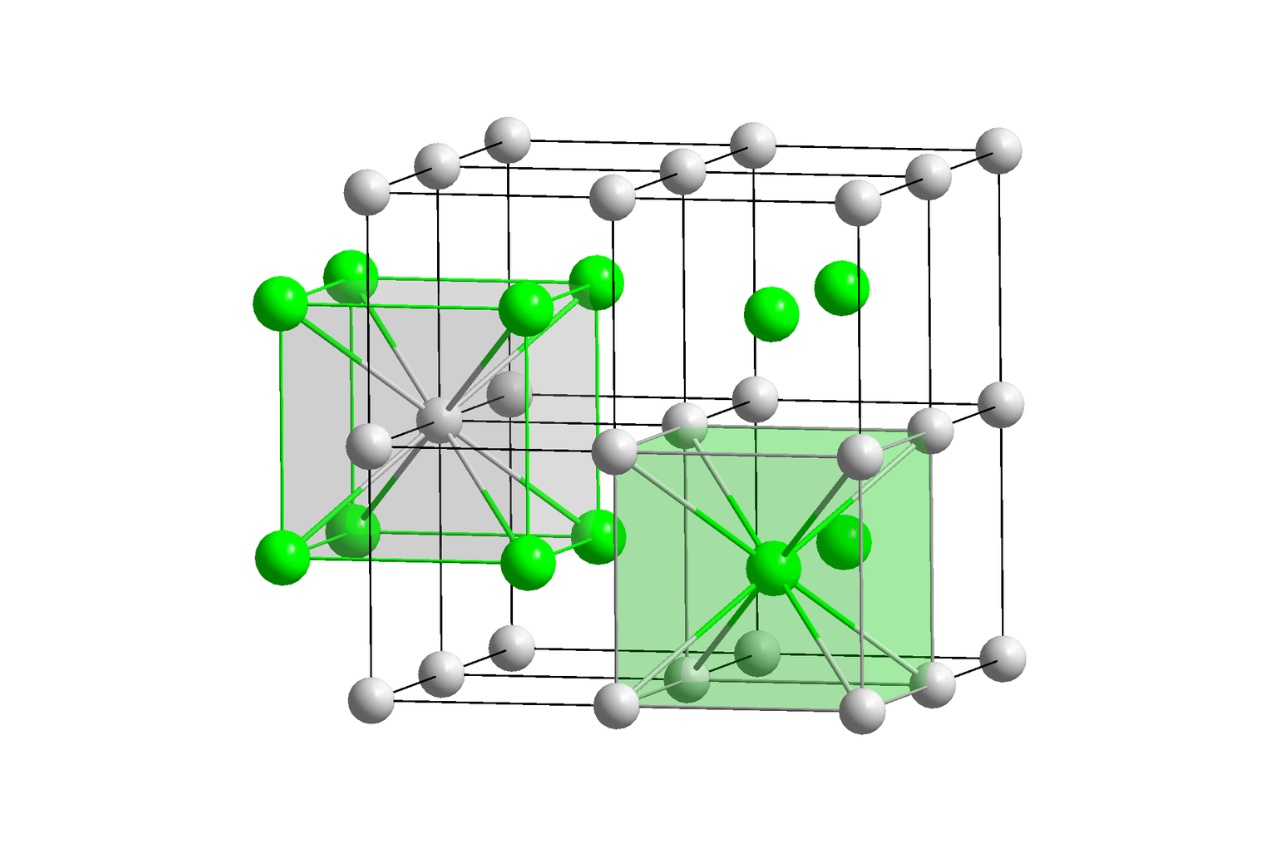
Crystals are not only beautiful to look at, but they also possess intricate structures known as crystal lattices. These lattices are formed by repeating patterns of atoms or molecules, giving crystals their unique properties and shapes. In the field of chemistry, the study of crystal lattices is of utmost importance, as it helps us understand the fundamental nature of matter and its behavior.
In this article, we will explore 9 captivating facts about crystal lattices. From the mesmerizing arrangement of atoms to the influence of lattice structures on physical properties, these facts will deepen your appreciation for the wonders of chemistry. So, let’s dive into the fascinating world of crystal lattices and uncover the secrets they hold!
Key Takeaways:
- Crystal lattice determines the physical and chemical properties of crystals, influencing their hardness, melting point, conductivity, and optical characteristics. It’s like the secret recipe that makes each crystal unique!
- X-ray diffraction helps scientists peek into crystal lattice structures, revealing the atomic arrangement within crystals. It’s like using a special X-ray vision to see the hidden patterns in crystals!
The Basic Structure of Crystal Lattice
Crystal lattice refers to the repetitive arrangement of atoms or molecules in a crystalline solid. It forms a three-dimensional pattern with a specific geometric shape. Each lattice point represents an atom or ion, and the arrangement determines the physical properties of the crystal.
Crystals Exist in Different Shapes and Structures
Crystal lattices can take on various shapes and structures, such as cubic, tetragonal, orthorhombic, and hexagonal. These different structures result from variations in the arrangement of atoms or molecules within the crystal lattice.
Crystal Lattice Determines the Physical and Chemical Properties of Crystals
The arrangement and bonding of atoms within the crystal lattice directly influence the physical and chemical properties of crystals. These properties include hardness, melting point, conductivity, and optical characteristics.
Symmetry Plays a Crucial Role in Crystal Lattice
Crystal lattices exhibit different levels of symmetry, which can be described using crystallographic notation. Symmetry operations, such as rotation, translation, and reflection, allow for the repetition of the lattice pattern.
X-Ray Diffraction Reveals Crystal Lattice Structures
X-ray diffraction is a powerful technique used to determine the atomic arrangement within crystal lattices. By analyzing the diffraction pattern created when X-rays pass through a crystal, scientists can deduce the crystal lattice structure.
Solid-State Chemistry Relies on Crystal Lattice Analysis
Crystal lattice analysis is essential in the field of solid-state chemistry. It helps scientists understand the relationship between the structure of a crystal lattice and its properties, leading to the development of new materials with specific functions.
Defects in Crystal Lattice Affect Crystal Properties
Crystal lattices can contain defects, such as vacancies, interstitials, or dislocations. These defects can significantly influence the mechanical, electrical, and thermal properties of crystals, making them useful for various applications.
Crystal Lattices Demonstrate Translational Symmetry
One of the key characteristics of crystal lattices is translational symmetry. This means that the lattice pattern repeats itself in all directions, resulting in a regular and ordered arrangement of atoms or molecules throughout the crystal.
Crystal Lattices Have Infinite Extension
An intriguing aspect of crystal lattices is that they have infinite extension in space. The arrangement of atoms or molecules continues infinitely in all directions, although in reality, the crystal may be confined to a finite size.
Conclusion
Crystal lattices are fascinating structures that play a crucial role in the field of chemistry. Understanding the intricacies of crystal lattice arrangements helps us comprehend the properties and behavior of various substances. In this article, we have explored nine captivating facts about crystal lattices, shedding light on their significance and complexity.
We have learned how crystal lattices determine the shape and symmetry of crystals and how they influence properties such as transparency, conductivity, and hardness. We have also discovered how different materials exhibit different types of crystal lattice structures and how defects in the lattice can impact their overall characteristics.
Exploring crystal lattices opens up a world of possibilities and applications in various scientific fields, including materials science, solid-state physics, and drug discovery. By delving deeper into the structure and properties of crystal lattices, scientists can uncover new insights that contribute to technological advancements and improve our understanding of the natural world.
FAQs
Q: What is a crystal lattice?
A: A crystal lattice is a three-dimensional arrangement of atoms, ions, or molecules in a crystal. It determines the structure, properties, and behavior of the material.
Q: How are crystal lattices formed?
A: Crystal lattices are formed through the repeated stacking of identical repeating units called unit cells. These unit cells are arranged in a specific pattern to create the lattice structure.
Q: What is the significance of crystal lattice in chemistry?
A: Crystal lattices play a crucial role in understanding the properties of materials. They provide insights into factors such as symmetry, thermal expansion, electrical conductivity, and optical behavior.
Q: What are the different types of crystal lattice structures?
A: The most common types of crystal lattice structures include cubic, tetragonal, orthorhombic, rhombohedral, monoclinic, and triclinic lattices, each with unique arrangements and symmetry.
Q: Can defects occur in crystal lattices?
A: Yes, defects such as vacancies, impurities, or dislocations can occur in crystal lattices. These defects can affect the material’s mechanical, electrical, and optical properties.
Q: How do crystal lattices determine a material’s transparency?
A: The arrangement of atoms or molecules in a crystal lattice can determine how light interacts with the material. The presence of certain lattice structures may allow light to pass through, making the material transparent.
Q: Are all materials crystalline and have a crystal lattice?
A: No, not all materials are crystalline. Some materials, such as glass or amorphous solids, do not have a regular crystal lattice structure.
Q: Can crystal lattice structures be modified?
A: Crystal lattice structures can be modified through processes such as doping, alloying, or applying external conditions like temperature and pressure. These modifications can alter the material’s properties.
Q: What are some practical applications of crystal lattices?
A: Crystal lattices have practical applications in various industries, including electronics, materials engineering, pharmaceuticals, and nanotechnology. They are used in the development of semiconductors, drugs, and advanced materials.
Crystal lattices captivate with their intricate structures and properties, but there's even more to explore in the world of crystallography. Delve into the fascinating field of crystallography and its various aspects. Learn about the intriguing concept of vacancy defects and how they influence crystal behavior. Discover the mind-boggling facts surrounding point defects and their impact on material properties. Continue your journey through the captivating realm of crystals and uncover the secrets they hold.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
