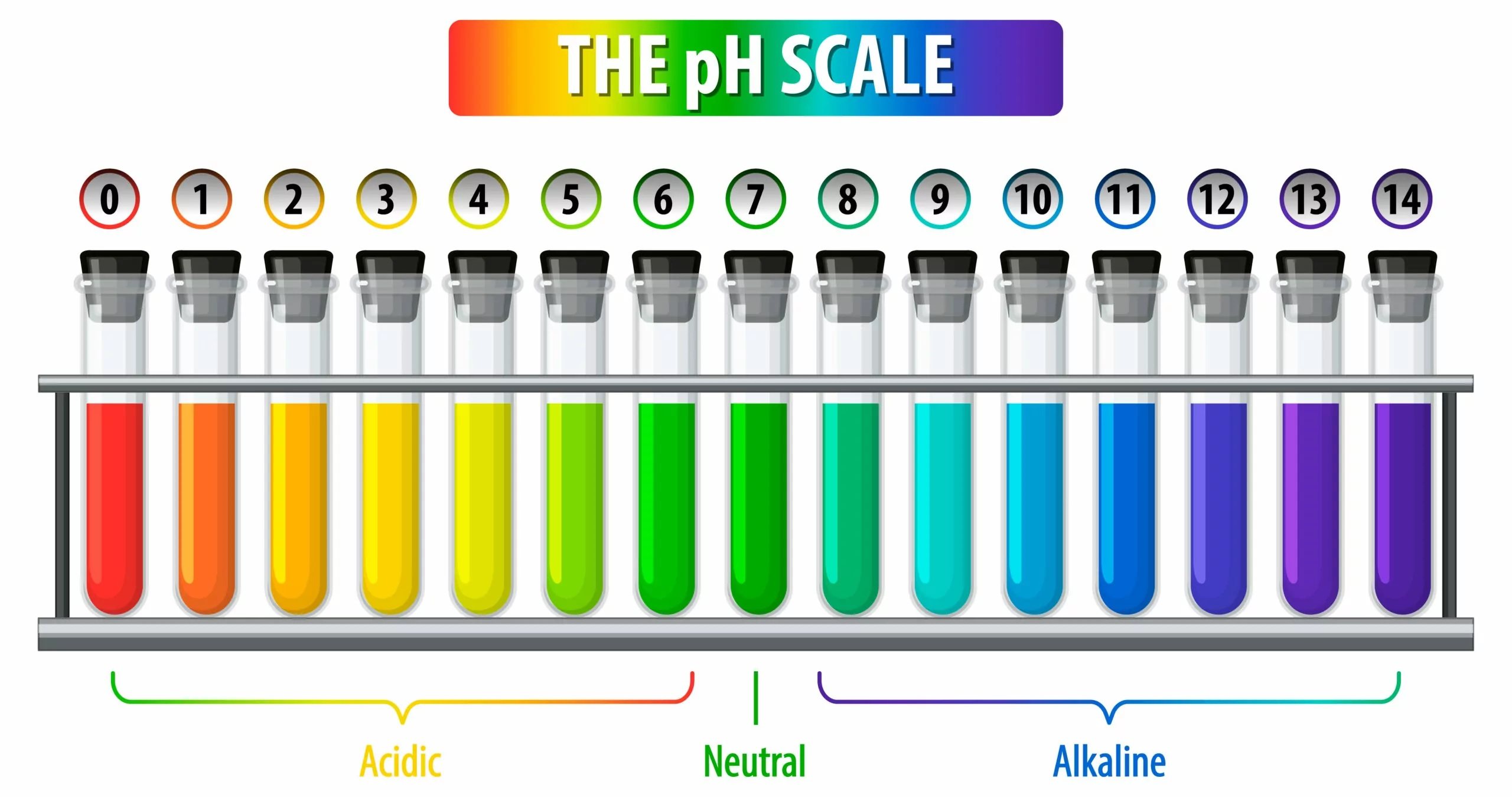
The pH scale is a fascinating concept in the field of chemistry that helps us understand the acidity or alkalinity of a substance. It is a logarithmic scale that ranges from 0 to 14, with 7 being considered neutral. Anything below 7 is acidic, while anything above 7 is alkaline. The pH scale is used to measure the concentration of hydrogen ions in a solution, indicating its level of acidity or basicity.
In this article, we will explore 15 mind-blowing facts about the pH scale that will leave you amazed at the intricacies of this important concept. From the origins of the pH scale to unusual applications in everyday life, we will delve into various intriguing aspects that highlight the significance of understanding pH in the world of chemistry. So, let’s dive in and uncover some incredible facts that will deepen our appreciation for the pH scale!
Key Takeaways:
- The pH scale measures acidity or alkalinity on a scale from 0 to 14, with 7 being neutral. It’s logarithmic, so each whole number change represents a tenfold difference in acidity or alkalinity.
- Understanding pH helps us grasp the acidity of everyday items like lemons and coffee, regulate pool water, and even impact the taste of food. It’s a crucial concept in chemistry that affects many aspects of our lives.
The pH scale ranges from 0 to 14.
At the center of the pH scale is 7, which is considered neutral. Solutions with a pH less than 7 are acidic, while those with a pH greater than 7 are alkaline or basic.
The pH scale is logarithmic.
This means that each whole number change on the pH scale represents a tenfold difference in acidity or alkalinity. For example, a solution with a pH of 2 is ten times more acidic than a solution with a pH of 3.
Lemons are highly acidic.
With a pH of around 2, lemons are one of the most acidic fruits. Their high acidity gives them their distinctive sour taste.
Vinegar is also acidic.
Vinegar, often used in cooking and cleaning, has a pH of around It gets its acidity from the acetic acid present in it.
Water has a neutral pH.
Pure water has a pH of 7, making it neither acidic nor alkaline. However, impurities and dissolved substances can alter its pH.
The pH of soda is highly acidic.
Popular carbonated beverages like soda have a pH level as low as 2.5 to 3.5, which is extremely acidic. This acidity can have negative effects on dental health.
Human blood has a slightly alkaline pH.
The pH of human blood is typically around 7.4, indicating a slightly alkaline nature. This stable pH is crucial for our bodies to function properly.
The pH of pool water must be carefully regulated.
Pool water should ideally have a pH level of 7.4 to 7.6 to ensure comfort and prevent the growth of harmful bacteria. pH levels outside of this range can cause skin and eye irritation.
The pH of black coffee is acidic.
Black coffee typically has a pH level around 5, making it mildly acidic. Adding milk or cream can neutralize its acidity.
The pH of the ocean is slightly basic.
The average pH of the ocean is around 8.1, reflecting its slightly alkaline nature. However, rising carbon dioxide levels are causing ocean acidification, which can have damaging effects on marine life.
pH can affect plant growth.
The pH of the soil directly impacts the availability of essential nutrients for plants. Different plants thrive best at specific pH levels.
pH levels can be tested using indicators.
Indicators such as litmus paper, pH paper, or pH meters can be used to measure the pH of a solution accurately.
pH can impact the taste of food.
The pH of ingredients can affect the taste and texture of food. For example, a higher pH level in dough can lead to a lighter and fluffier bread.
pH levels in the body are regulated by buffers.
Buffers in the body help maintain the pH balance, preventing drastic changes that could be harmful. They help stabilize the pH of bodily fluids.
pH has an impact on swimming pool chlorine effectiveness.
The effectiveness of chlorine in swimming pools is influenced by the pH level. If the pH is too high or too low, it can hinder the chlorine’s ability to sanitize the water properly.
These 15 mind-blowing facts about the pH scale demonstrate its significance in various aspects of our lives. From understanding the acidity of common kitchen ingredients to regulating the pH of our bodies and maintaining the health of our pool water, the pH scale plays a crucial role. By grasping the concept of pH, we can appreciate the delicate balance that exists in the chemical world.
Conclusion
In conclusion, the pH scale is an essential tool in the field of chemistry. It allows us to understand the acidity or alkalinity of a substance and plays a crucial role in various industries and everyday life. Through this article, we have explored 15 mind-blowing facts about the pH scale, from its origins to its applications. We have learned about the logarithmic nature of the scale, the importance of pH in aquatic environments, and the impact of pH on living organisms. Understanding the pH scale opens doors to a deeper understanding of chemistry and its practical implications. So, next time you come across the concept of pH, remember these fascinating facts and appreciate the ubiquitous presence and significance of pH in our world.
FAQs
1. What does pH stand for?
pH stands for “power of hydrogen.”
2. What is the pH range?
The pH scale ranges from 0 to 14, with 0 being highly acidic, 7 being neutral, and 14 being highly alkaline.
3. How is pH measured?
pH is measured using a pH meter or pH test strips. The meter determines the concentration of hydrogen ions in a solution, while the test strips indicate the pH value based on color changes.
4. What are some examples of acid and alkaline substances?
Examples of acid substances include lemon juice, vinegar, and battery acid. Alkaline substances include baking soda, soap, and bleach.
5. Why is pH important in agriculture?
pH affects the availability of nutrients in the soil, which directly impacts plant growth. Different crops have different pH requirements, and maintaining appropriate pH levels ensures optimal growing conditions.
Fascinating facts about pH abound, but that's just the beginning! Dive deeper into pH measurement techniques for even more astounding insights. Acidity plays a crucial role in countless chemical reactions, as you'll discover in our exploration of Bronsted-Lowry acids. Alkalinity is equally important, and our article on acid-base indicators will reveal extraordinary facts about how these substances help us identify pH levels. Keep learning and satisfy your curiosity with these captivating reads!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
