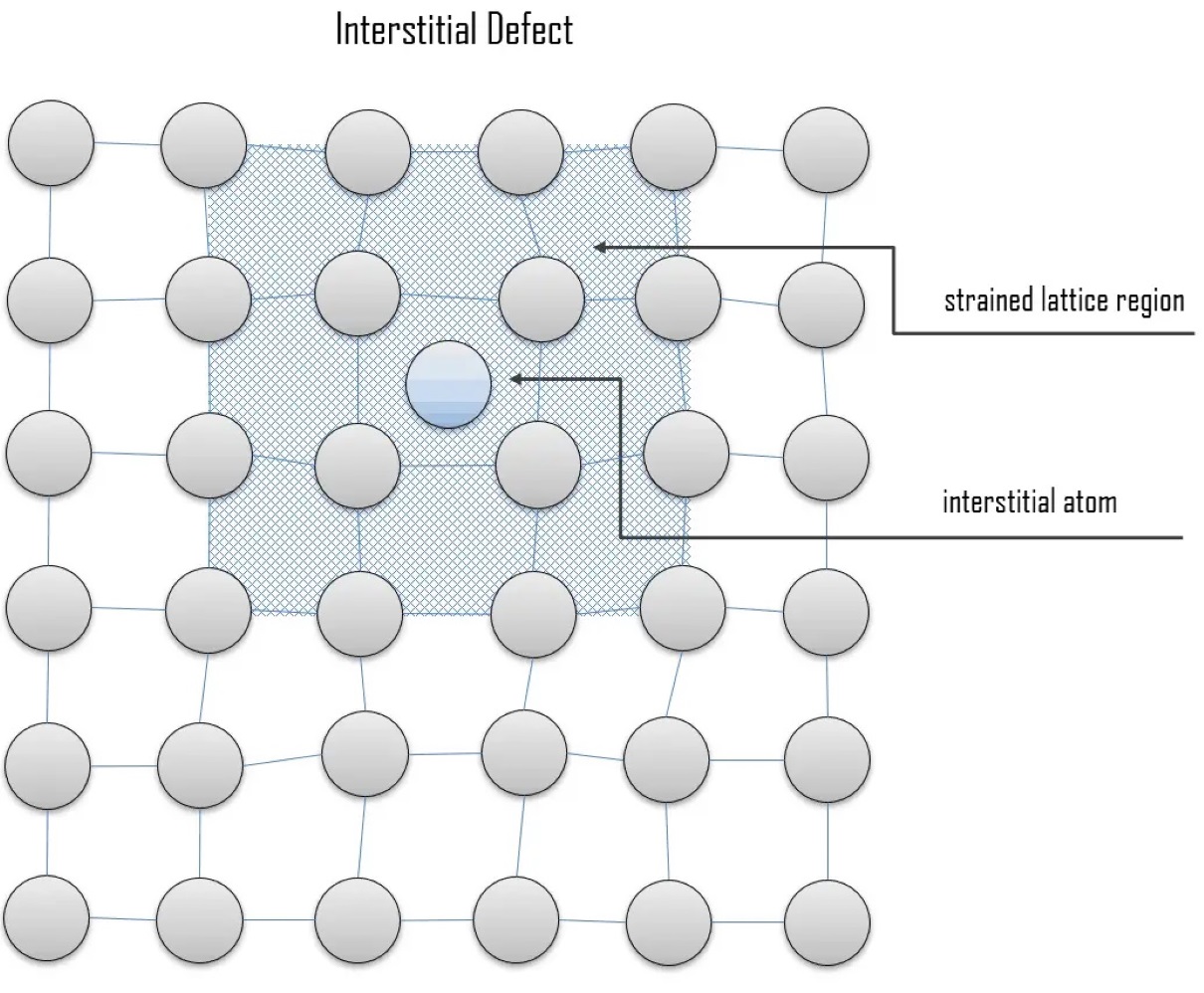
Interstitial defects are a fascinating and often perplexing aspect of chemistry. These defects occur when atoms or ions occupy the spaces between the regular lattice sites in a crystal structure. While they may seem like imperfections at first glance, interstitial defects play a crucial role in determining the physical and chemical properties of materials.
In this article, we will explore 19 mind-blowing facts about interstitial defects, shedding light on their significance and impact. From their role in enhancing mechanical strength to their influence on the electrical conductivity of materials, interstitial defects have a wide range of implications in various fields, including metallurgy, solid-state physics, and materials science.
So, fasten your seatbelts and get ready to embark on a journey through the intriguing world of interstitial defects, where the seemingly vacant spaces within crystal structures hold surprising and awe-inspiring secrets.
Key Takeaways:
- Interstitial defects can change the way materials behave and look, like making gemstones colorful or affecting how well things conduct heat and electricity.
- Scientists can use interstitial defects to create new materials with special properties for different uses, like making stronger or more colorful things.
Interstitial Defect occurs in crystalline structures.
Interstitial Defect is a phenomenon that happens within crystalline structures, where foreign atoms or molecules occupy spaces between lattice points.
It can affect the properties of materials.
The presence of interstitial defects can significantly impact the mechanical, electrical, and optical properties of materials, altering their behavior and functionality.
Interstitial defects can enhance or decrease material strength.
Depending on the nature and arrangement of the interstitial defects, they can either strengthen or weaken the material. Introducing interstitial atoms can create obstacles for dislocations, leading to increased strength.
They can cause lattice distortion.
When foreign atoms occupy interstitial sites, they disrupt the regular arrangement of atoms within the lattice, causing lattice distortion and affecting the overall crystal structure.
Interstitial defects can influence diffusion rates.
The presence of interstitial defects can impede or facilitate the movement of atoms within the crystal lattice, affecting the diffusion rates of different species in the material.
They can lead to coloration in gemstones.
In some gemstones, interstitial defects can introduce impurities that alter their color. For example, the presence of chromium atoms as interstitial defects in corundum produces the vibrant red color of ruby.
Interstitial defects can result in point defects.
In certain cases, the presence of interstitial defects can lead to the formation of point defects, such as vacancies or self-interstitial atoms within the lattice structure.
They can influence the electronic properties of materials.
Interstitial defects can introduce additional energy levels within the band structure of materials, affecting their electronic properties and conductivity.
The size of interstitial atoms affects the defect characteristics.
The size and nature of the atoms occupying interstitial sites play a crucial role in determining the specific characteristics and effects of the interstitial defect.
Interstitial defects can be intentional or unintentional.
In some cases, interstitial defects are deliberately introduced during the synthesis or processing of materials for specific purposes, while in others, they may occur naturally or unintentionally.
Interstitial defects can affect the diffusion of gases.
The presence of interstitial defects can influence the diffusion behavior of gases in materials, which has significant implications in fields such as catalysis and gas separation.
They can result in altered surface properties.
The presence of interstitial defects near the surface of a material can significantly impact its surface properties, such as adsorption, reactivity, and corrosion resistance.
Interstitial defects can affect the thermal conductivity of materials.
The incorporation of interstitial atoms can disrupt the regular heat transfer pathways within a material, leading to changes in its thermal conductivity.
They can lead to changes in material phase transitions.
Interstitial defects can influence the kinetics and temperature at which phase transitions occur in materials, leading to altered material behavior under different conditions.
Interstitial defects can be key to designing novel materials.
By manipulating the presence and arrangement of interstitial defects, scientists and engineers can create new materials with tailored properties for various applications.
They can form complexes with other defects.
Interstitial defects can interact and form complexes with other defects within the crystal lattice, further altering the material’s behavior and characteristics.
Interstitial defects can affect the optical properties of materials.
Changes in the crystal structure due to interstitial defects can affect the absorption, reflection, and transmission of light, resulting in variations in a material’s optical properties.
They can contribute to strain in materials.
The incorporation of interstitial atoms can cause lattice strain, leading to internal stress within the material and potential changes in its mechanical properties.
Interstitial defects play a role in radiation damage.
In materials exposed to radiation, interstitial defects can be created due to the displacement of atoms, leading to radiation damage and degradation of material properties.
Conclusion
In conclusion, interstitial defects are fascinating phenomena that occur in the field of chemistry. These defects, characterized by the presence of foreign atoms in the crystal lattice, can have a significant impact on the properties and behavior of substances. Throughout this article, we have explored 19 mind-blowing facts about interstitial defects, shedding light on their effects on various materials and their potential applications.We have learned that interstitial defects can enhance the strength and hardness of materials, leading to the development of stronger alloys. These defects can also influence the electrical conductivity, thermal properties, and even the color of substances. Understanding interstitial defects is crucial for improving the performance of materials in various industries, such as aerospace, automotive, and electronics.Overall, the study of interstitial defects provides valuable insights into the intricate nature of chemical structures and their impact on material properties. Exploring this fascinating topic further can lead to exciting advancements in the realm of chemistry and open up new possibilities for innovation and technological breakthroughs.
FAQs
1. What are interstitial defects?
Interstitial defects are imperfections in the crystal lattice of a material where foreign atoms occupy spaces between the regular lattice points.
2. How do interstitial defects influence material properties?
Interstitial defects can affect various material properties such as strength, hardness, electrical conductivity, and thermal properties by introducing different atomic species into the crystal lattice.
3. Are interstitial defects always detrimental?
No, interstitial defects can have both positive and negative effects on material properties. In some cases, they can enhance certain properties, such as strength, while in other cases, they may reduce desired characteristics.
4. Can interstitial defect engineering be controlled?
Yes, interstitial defect engineering involves deliberately introducing specific foreign atoms to modify the material properties. By controlling the type and concentration of interstitial defects, their effects can be tailored for specific applications.
5. Are there any real-world applications of interstitial defects?
Yes, interstitial defects have numerous applications. For example, interstitial doping is used in semiconductor technology to improve the electrical performance of transistors and other electronic devices.
6. Can interstitial defects be removed or repaired?
In some cases, interstitial defects can partially or completely anneal during heat treatment processes, resulting in the removal or repair of the defects. However, the complete elimination of interstitial defects can be challenging.
7. How does the presence of interstitial defects affect the color of materials?
When foreign atoms occupy interstitial sites, they can alter the electronic structure of the material, leading to changes in its optical properties. This can result in a change in color or the absorption and emission of light.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
