Acids are an essential part of our daily lives, playing a significant role in various fields such as chemistry, biology, and even food. These substances, known for their sour taste and ability to corrode certain materials, possess intriguing properties and applications. In this article, we will explore 18 fascinating facts about acids, shedding light on their nature, uses, and impact. So let’s dive in and uncover the secrets of acids!
What Are Acids?
Acids are substances that, when dissolved in water, release hydrogen ions (H+). They are characterized by their pH level, which ranges from 0 to 6.9, indicating their acidic nature. Acids can be found in both natural and synthetic forms, serving various purposes across different industries.
The pH Scale
The pH scale measures the acidity or alkalinity of a substance. It ranges from 0 to 14, with 0 being highly acidic, 7 neutral, and 14 highly alkaline. Acids fall below 7 on the pH scale, with lower numbers representing stronger acidity.
Common Types of Acids
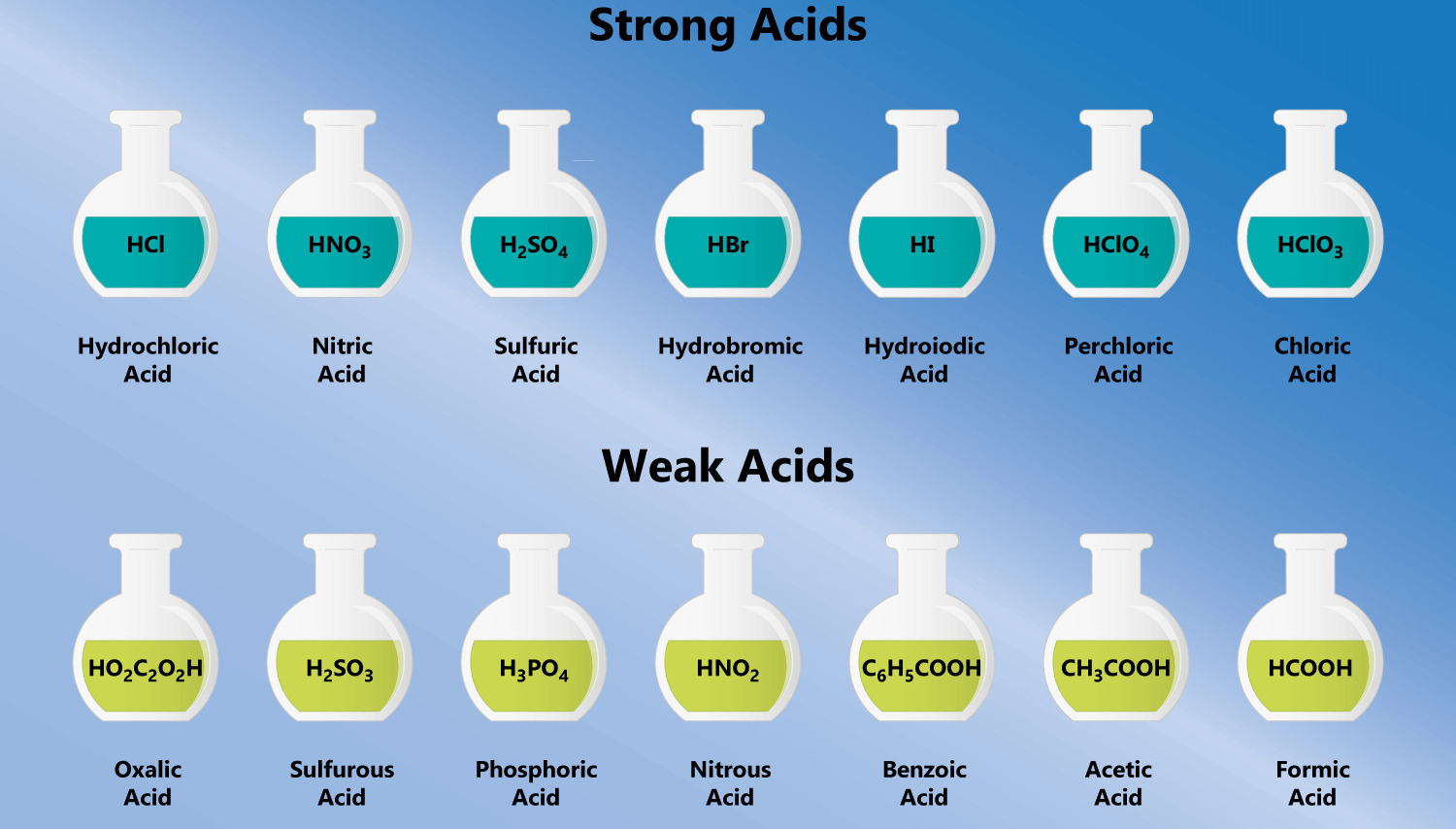
There are several types of acids, each with its own unique properties and applications. Some common examples include:
Hydrochloric Acid (HCl): Found in our stomachs, it aids in the digestion of food.
Sulfuric Acid (H2SO4): Widely used in the production of fertilizers, detergents, and dyes.
Citric Acid (C6H8O7): Present in citrus fruits and used as a flavor enhancer in food and beverages.
Acetic Acid (CH3COOH): The main component of vinegar and used in the production of plastics and textiles.
Natural Acids in Foods
Acids are prevalent in various foods, contributing to their taste and preservation. Some natural acids found in everyday food items include:
Lactic Acid: Present in yogurt, pickles, and sourdough bread.
Tartaric Acid: Found in grapes, bananas, and wine.
Malic Acid: Abundant in apples, cherries, and tomatoes.
Acid Rain
Acid rain is a result of air pollution caused by emissions of sulfur dioxide and nitrogen oxide. These gases react with water vapor in the atmosphere, forming sulfuric and nitric acid. When it precipitates, it can have detrimental effects on the environment, including damage to vegetation, aquatic life, and infrastructure.
Corrosive Properties
One of the defining characteristics of acids is their corrosive nature. Strong acids can corrode metals, leading to the deterioration of structures and equipment. This property is utilized in industrial applications such as cleaning, etching, and metal refining.
Acid-Base Reactions
Acids and bases can undergo neutralization reactions, wherein an acid reacts with a base to form salt and water. This process is widely used in various chemical reactions, including the preparation of salts, medicines, and fertilizers.
Digestive Acids
The human stomach contains hydrochloric acid, which plays a vital role in the digestion of food. This powerful acid helps break down proteins, activates digestive enzymes, and kills harmful bacteria that may enter the digestive system.
Industrial Applications
Acids find extensive use in numerous industries. Sulfuric acid, for example, is utilized in the production of fertilizers, pigments, and detergents. Nitric acid is employed in the manufacturing of explosives and fertilizers, while citric acid is a common ingredient in the food and beverage industry.

Vitamin C – Ascorbic Acid
Vitamin C, also known as ascorbic acid, is an essential nutrient for humans. It acts as an antioxidant, aids in collagen synthesis, and boosts the immune system. Citrus fruits, strawberries, and leafy green vegetables are excellent sources of this vital acid.
Battery Acids
Lead-acid batteries, commonly used in vehicles, employ a sulfuric acid electrolyte to generate electricity through chemical reactions. These batteries are rechargeable and have been widely used for decades to power automobiles, boats, and backup power systems.
Acids in Skincare
Some acids, such as salicylic acid and glycolic acid, are utilized in skincare products. Salicylic acid helps in the treatment of acne by unclogging pores and reducing inflammation. Glycolic acid, on the other hand, is an exfoliant that helps remove dead skin cells and improve the overall texture of the skin.
Ant Sting and Formic Acid
When ants bite or sting, they inject venom-containing formic acid into their victims. This acid causes a burning sensation and can trigger an allergic reaction in some individuals. Formic acid derived its name from the Latin word “Formica,” meaning ant.
Acids in Photography
Photographic development relies on acids, particularly acetic acid and citric acid. These acids are used in developing solutions to control the pH levels and enhance the image development process.
Acidic and Alkaline Soils
Soil acidity or alkalinity affects plant growth and agriculture. Some plants thrive in acidic soil, while others prefer alkaline conditions. Soil tests are conducted to determine the pH level and take necessary measures, such as adding lime to raise the pH of acidic soils or sulfur to lower the pH of alkaline soils.
Acidic Aquatic Environments
Certain bodies of water, such as lakes or rivers, can become acidic due to pollution and natural processes. Acidic water has adverse effects on aquatic life, disrupting the ecosystem and threatening the survival of various species.
Acidic Household Cleaners
Many household cleaners, such as toilet bowl cleaners and descalers, contain acidic substances like hydrochloric acid or phosphoric acid. These acids help remove mineral deposits, stains, and grime from surfaces, making them an effective cleaning agent.
Acid Neutralizers
In case of accidental exposure to acids or acid burns, it is crucial to neutralize the acid quickly. Substances like baking soda (sodium bicarbonate) or calcium carbonate can be used to neutralize acids and prevent further damage.
Conclusion
With a wide range of applications and intriguing properties, acids continue to be an essential component in various aspects of our lives. By understanding their nature and potential, we can harness their power for the benefit of numerous industries and advancements in science and technology.
Frequently Asked Questions (FAQs)
Are all acids harmful to humans?
Not all acids are harmful to humans. In fact, our stomachs naturally produce hydrochloric acid to aid digestion. However, strong acids can be corrosive and cause harm if not handled properly.
How are acids used in batteries?
Acids, such as sulfuric acid, are used as electrolytes in lead-acid batteries to facilitate the chemical reactions that generate electrical energy.
Can acids be found in nature?
Yes, acids can be found in nature. Citric acid, for example, is naturally present in citrus fruits like oranges and lemons.
Do acids always taste sour?
Most acids have a sour taste, but not all sour-tasting substances are acids. Some artificial sweeteners can also have a sour taste.
Can acids be used for cleaning metals?
Yes, acids can be used to clean metals through a process called etching. However, caution must be exercised as strong acids can cause corrosion if not used properly.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
