Are you ready to explore the fascinating world of chemistry? Get ready to delve into the intriguing realm of vacancy defects! Vacancy defects are a captivating phenomenon that occur in various materials, from metals to crystals. These defects involve the presence of missing atoms or ions within the crystal lattice structure, creating unique properties and characteristics. In this article, we will uncover 10 unbelievable facts about vacancy defects that will leave you in awe of the wonders of chemistry. Prepare to be amazed as we explore the impact of vacancy defects on conductivity, magnetism, and even catalytic activity. So, let’s dive in and discover the incredible world of vacancy defects!
Key Takeaways:
- Vacancy defects are like tiny missing puzzle pieces in materials, affecting their properties in surprising ways. They can enhance electrical conductivity, influence diffusion, and even create new opportunities for innovation in materials science.
- Nature’s creative chaos leaves behind vacancy defects in materials, opening doors to new possibilities and challenges. Understanding and harnessing these imperfections is crucial for advancing technology and creating extraordinary materials.
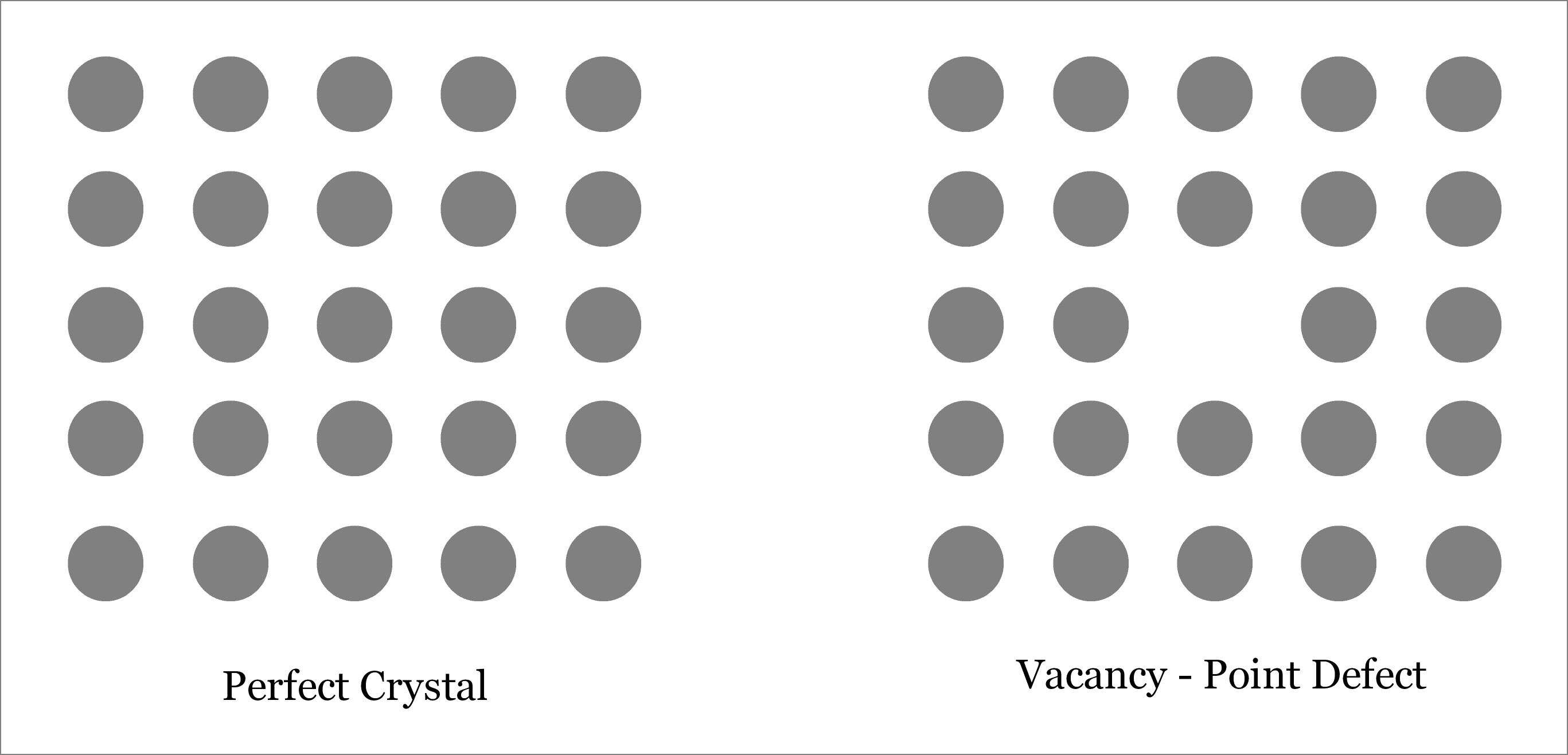
The Vacancy Defect: A Mysterious Imperfection
One of the most intriguing phenomena in materials science is the vacancy defect. These defects occur when an atom is missing from its expected position within a crystal lattice. It may seem like a simple absence, but the implications and consequences are far from ordinary.
Nature’s Imperfection
Did you know that vacancy defects are a natural occurrence in almost all materials? Even the most perfect crystals exhibit these imperfections at the atomic level. It’s as if nature purposely leaves behind a trace of its creative chaos within the ordered structure of matter.
The Perfect Vacancy Storm
Under certain conditions, vacancy defects can cluster together, creating what is known as a “perfect vacancy storm.” These clusters can significantly affect the material’s properties, leading to unexpected behavior and even spontaneous phase transitions.
Vacancy Defects and Electrical Conductivity
When it comes to electrical conductivity, vacancy defects play a crucial role. In some materials, the presence of these defects can enhance conductivity by providing additional pathways for electron movement. This phenomenon is exploited in various technological applications, from semiconductors to superconductors.
Vacancy Healing: Nature’s Repair Mechanism
Interestingly, vacancy defects are not permanent. Given the right conditions, these imperfections can be “healed” as atoms shift into vacant positions, restoring the crystal lattice’s integrity. This self-healing property of vacancy defects adds another layer of complexity to their behavior and impact on material properties.
Vacancy Defects and Diffusion
Diffusion, the movement of atoms through a material, can be greatly influenced by vacancy defects. The presence of these defects provides diffusion paths for atoms to move more freely, resulting in an enhanced diffusion rate. This mechanism is widely studied in fields such as metallurgy and materials engineering.
Vacancy Defects in Catalysts
Catalysts play a vital role in various chemical reactions, and vacancy defects can significantly impact their performance. These defects can affect the surface reactivity, adsorption properties, and overall catalytic activity of materials used as catalysts, making the study of vacancy defects essential in the field of catalysis.
Vacancy Defects and Optical Properties
Vacancy defects can also have fascinating effects on the optical properties of materials. By creating localized energy states within the crystal lattice, these defects can alter the absorption, emission, and reflection of light. This phenomenon is widely studied in the development of optoelectronic devices.
Vacancy Defects and Mechanical Properties
The presence of vacancy defects can significantly impact the mechanical properties of materials. These defects act as stress concentrators, reducing the material’s strength and altering its fracture behavior. Understanding the role of vacancy defects is crucial for designing materials with improved mechanical properties.
Vacancy Defects: A Window to New Possibilities
While vacancy defects may disrupt the perfect order within materials, they also open up new opportunities for innovation. Researchers are continuously exploring the potential of these defects to control and manipulate material properties, leading to the development of advanced materials with tailored characteristics.
Conclusion
Vacancy defects are fascinating to study and uncover. These imperfections in the crystal lattice structure of materials can have significant effects on their physical and chemical properties. From influencing the conductivity of semiconductors to altering the color of gemstones, vacancy defects play a crucial role in various fields of science and technology.
By understanding the nature and behavior of vacancy defects, researchers can develop new materials with enhanced properties or find innovative ways to manipulate these defects for practical applications. As we delve deeper into the realm of materials science, we continue to uncover the hidden wonders of vacancy defects, revolutionizing industries and pushing the boundaries of our knowledge.
Exploring the realms of vacancy defects has just scratched the surface of their potential. As research progresses, we can anticipate even more astonishing discoveries that will shape the future of materials and technology.
FAQs
1. What exactly is a vacancy defect?
A vacancy defect refers to an imperfection in a crystal lattice structure where an atom or ion is missing from its expected position. It creates an empty space or “vacancy” in the lattice, disrupting the perfect arrangement of atoms or ions.
2. How do vacancy defects occur?
Vacancy defects can occur during the crystal growth process when atoms or ions are not properly incorporated into the lattice structure. They can also arise due to thermal or irradiation-induced displacements, chemical reactions, or even mechanical stresses.
3. What are the effects of vacancy defects?
Vacancy defects can significantly impact the properties of materials. For example, it can alter electrical conductivity, optical properties, and mechanical strength. Additionally, vacancy defects can affect the diffusion of atoms and the catalytic activity of materials.
4. Can vacancy defects be intentionally created?
Yes, scientists can intentionally create vacancy defects using various techniques like ion implantation, thermal treatments, and chemical reactions. By controlling the number and distribution of vacancy defects, they can modify the properties of materials to suit specific applications.
5. Are vacancy defects only found in inorganic materials?
No, vacancy defects can occur in both inorganic and organic materials. In inorganic materials like ceramics and semiconductors, vacancy defects are more commonly studied, but they can also exist in organic molecules and polymers.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


