Titration curve is a fundamental concept in chemistry that plays a crucial role in determining the unknown concentration of a solution. It involves the gradual addition of a titrant to an analyte until the equivalence point is reached. This process allows chemists to accurately measure the amount of substance in a solution and understand its properties.
In this article, we are going to explore 10 mind-blowing facts about titration curves that will enhance your understanding of this essential analytical technique. From the different types of titration curves to the significance of the inflection point, prepare to delve into the world of titration and discover some fascinating insights.
Key Takeaways:
- Titration curves show how pH changes during a chemical reaction. They help scientists understand acid-base reactions and determine the strength of acids.
- The shape of a titration curve can reveal the nature of the reaction and the presence of polyprotic acids. It’s like a secret code that chemists use to unlock the mysteries of chemicals!
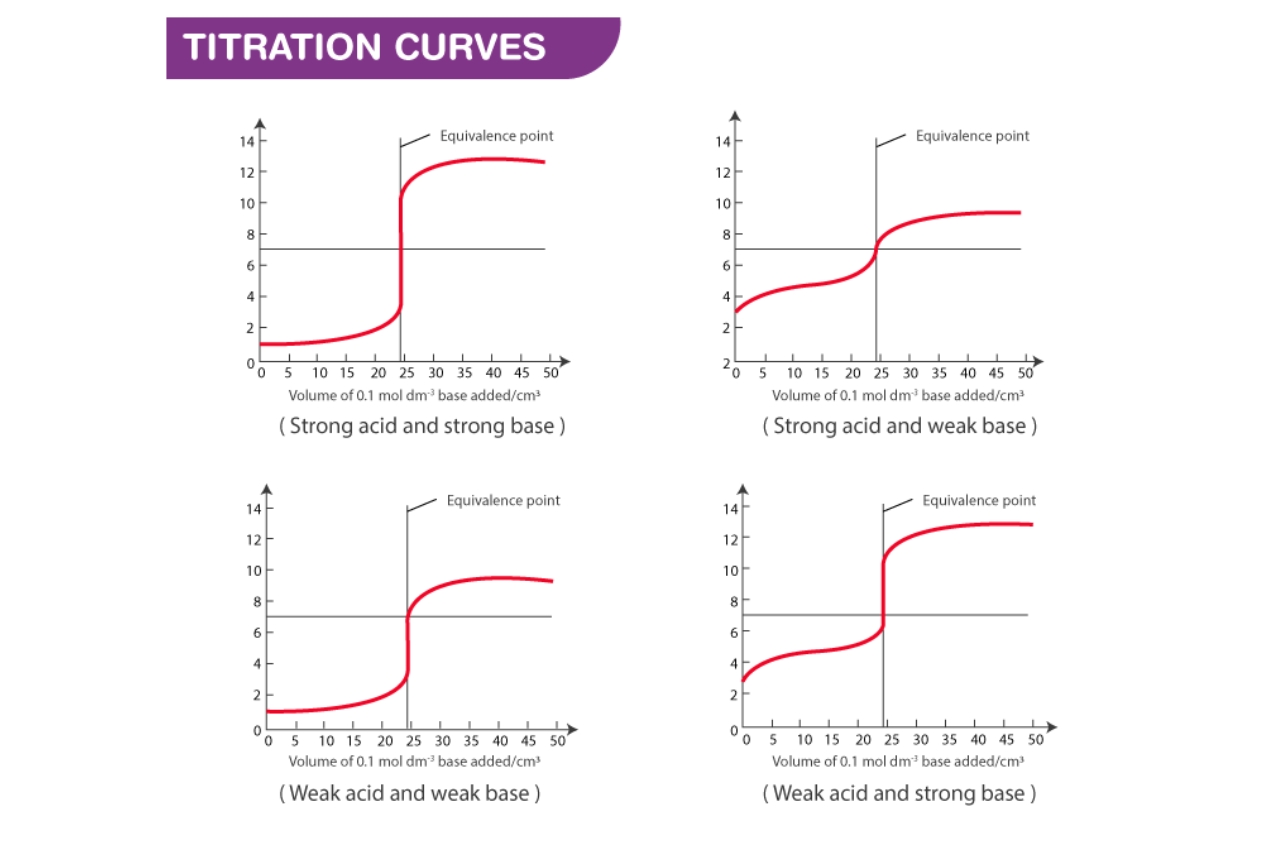
The titration curve is a graphical representation of a chemical reaction.
The key observation during a titration is the change in pH as a reactant is added to a solution. The plot of pH against the volume of the added reactant is called the titration curve.
The shape of the titration curve depends on the nature of the acid-base reaction.
Depending on the type of reaction, the titration curve may exhibit various shapes, such as sharp or gradual slopes, inflection points, or overlapping curves.
The equivalence point represents the stoichiometrically equivalent amounts of acid and base.
At the equivalence point, the moles of acid and base are in a 1:1 ratio, resulting in neutralization and a pH of 7 for a strong acid-strong base titration.
Titration curves can also be used to determine the pKa value of weak acids.
By analyzing the pH changes at different points on the titration curve, one can calculate the pKa value of a weak acid and gain insight into its acid strength.
The pH at the half-equivalence point can be used to determine the pKa of a weak acid.
At the half-equivalence point, which is halfway between the initial pH and the equivalence point, the concentration of the weak acid and its conjugate base are equal, allowing for pKa determination.
Titration curves can be used to identify the presence of polyprotic acids or bases.
When a polyprotic acid or base is titrated, multiple inflection points can be observed on the titration curve, corresponding to the successive deprotonation or protonation steps.
Indicators can be used to visually determine the endpoint of a titration.
Indicators change color when the solution reaches a certain pH range, indicating the completion of the titration. Common indicators include phenolphthalein, bromothymol blue, and methyl orange.
Titration curves can vary depending on temperature and ionic strength.
Changes in temperature or ionic strength of the solution can influence the shape and position of the titration curve, highlighting the importance of controlling these factors during titration experiments.
The slope of the titration curve is steepest at the equivalence point.
The steep slope indicates a rapid change in pH with the addition of the reactant, reflecting the highly acidic or basic nature of the solution at the equivalence point.
Titration curves are widely used in various fields, including analytical chemistry and pharmaceutical research.
Titration curves provide valuable insights into the behavior of acids and bases, allowing scientists to determine unknown concentrations, characterize unknown compounds, and monitor the progress of chemical reactions.
Conclusion
In conclusion, the titration curve is an essential tool in the field of chemistry, providing valuable insights into chemical reactions and determining the properties of substances. By plotting the pH against the volume of the titrant, scientists can analyze various aspects of a reaction, such as the equivalence point, the endpoint, and the nature of the reactants.Understanding the titration curve allows chemists to determine the concentration of unknown substances, identify the acidity or alkalinity of a solution, and study the behavior of weak acids and bases. The shape of the curve provides information about the ions involved in the reaction and their equilibrium concentrations.By exploring these ten mind-blowing facts about the titration curve, we have gained a deeper appreciation for its significance in the world of chemistry. From the concept of buffering to the impact of indicators, the titration curve offers a wealth of knowledge that continues to unravel the mysteries of chemical reactions.
FAQs
1. What is a titration curve?
A titration curve is a graphical representation showing the pH changes of a solution as a titrant is added. It helps to determine various aspects of a reaction, such as the equivalence point and the nature of the reactants.
2. How is a titration curve plotted?
A titration curve is plotted by measuring the pH of the solution at different points during the titration process and plotting it against the volume of the titrant added.
3. What is the equivalence point in a titration curve?
The equivalence point is the point in a titration where stoichiometrically equivalent amounts of the reactants have reacted. It is indicated by a sharp change in the pH of the solution.
4. What is the significance of the endpoint in a titration curve?
The endpoint is the point in a titration where the desired reaction has just completed. It is usually determined by using an indicator or a pH meter.
5. How does a titration curve help determine the concentration of unknown substances?
By comparing the shape and characteristics of the titration curve of an unknown substance with those of known substances, chemists can determine its concentration.
6. What role do indicators play in a titration curve?
Indicators are substances that change color at different pH values. They help identify the endpoint and provide visual cues during titration.
7. Can a titration curve be used to study the behavior of weak acids and bases?
Yes, a titration curve can provide insights into the behavior of weak acids and bases. The shape of the curve differs from that of strong acids and bases due to the presence of weak conjugate acid-base pairs.
8. What is the concept of buffering in a titration curve?
Buffering refers to the ability of a solution to resist changes in pH when small amounts of acid or base are added. Buffer solutions show a flat region in the titration curve, indicating their buffering capacity.
9. Are there any limitations to the titration curve?
Yes, the titration curve assumes ideal conditions, which may not always be the case in real-world applications. Factors such as temperature, concentration, and presence of impurities can affect the accuracy of the curve.
10. How can the titration curve be applied in various fields of chemistry?
The titration curve finds applications in areas such as pharmaceuticals, environmental analysis, food chemistry, and determining the purity of substances. It plays a crucial role in research, analysis, and quality control processes.
Titration curves offer a fascinating glimpse into the world of chemistry, revealing the intricate dance between acids and bases. But there's more to explore! Delve deeper into the complexities of acid-base reactions with our article on 20 extraordinary facts about acid-base titration curves. From the role of indicators to the impact of temperature, you'll gain a comprehensive understanding of this essential analytical technique. So, whether you're a chemistry enthusiast or simply curious about the science behind titrations, our article has something for everyone. Join us on this educational journey and expand your knowledge today!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
