Self-diffusion, a fundamental concept in the field of chemistry, plays a vital role in various processes occurring within substances. It refers to the movement of individual molecules within a substance, without the aid of any external forces. Understanding self-diffusion is essential as it helps us comprehend the behavior of matter at a molecular level.
Intriguingly, self-diffusion presents us with a plethora of captivating facts that shed light on the inherent nature of substances and their atomic interactions. From its role in everyday phenomena to its significance in cutting-edge scientific research, self-diffusion continues to amaze and enthrall chemists worldwide.
In this article, we will explore 16 fascinating facts about self-diffusion, delving into its principles, applications, and the mesmerizing insights it provides into the microscopic world. So, let’s embark on this journey into the realm of self-diffusion and discover the wonders it holds.
Key Takeaways:
- Self-diffusion is the spontaneous movement of particles without outside influence, affecting everything from the flow of liquids to the delivery of drugs in our bodies. It’s like a natural dance that shapes the world around us.
- Understanding self-diffusion helps scientists create better materials and drug delivery systems. It’s like uncovering the secret to making things move faster and more efficiently, from tiny particles to life-saving medications.
What is self-diffusion?
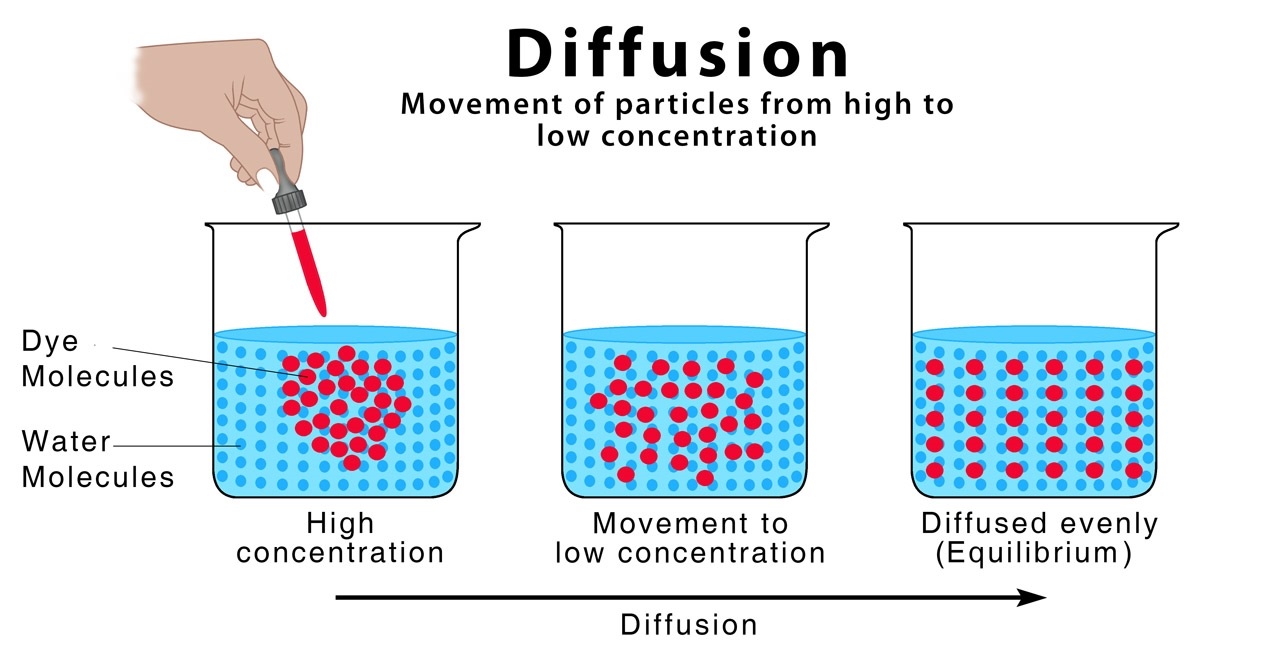
Self-diffusion refers to the spontaneous movement or spreading of particles through a medium without the influence of external forces. It occurs when molecules or atoms of a substance move randomly from regions of higher concentration to regions of lower concentration.
Diffusion is an essential process in nature.
From the movement of gases in the atmosphere to the transport of nutrients in living organisms, self-diffusion plays a vital role in various natural phenomena. Understanding the mechanisms and properties of self-diffusion is crucial in numerous scientific fields, including chemistry, physics, and biology.
Self-diffusion occurs in all states of matter.
Whether it’s the diffusion of molecules in a gas, the movement of ions in a liquid, or the migration of atoms in a solid, self-diffusion is a universal phenomenon that can be observed in gases, liquids, and solids.
Diffusion is influenced by temperature.
Increasing the temperature of a substance enhances the rate of self-diffusion. This is because higher temperatures provide more energy to the particles, allowing them to move more rapidly and cover greater distances.
The size and shape of particles affect self-diffusion.
Smaller particles tend to diffuse more rapidly than larger ones due to their increased mobility. Additionally, particles with irregular shapes experience hindered diffusion compared to those with regular shapes.
Self-diffusion is governed by the concentration gradient.
The concentration gradient, which refers to the difference in concentration between two regions, drives the process of self-diffusion. Molecules or atoms naturally move from areas of high concentration to areas of low concentration, seeking equilibrium.
Diffusion can be influenced by external factors.
Factors such as pressure, electric fields, and surface area can affect the rate of self-diffusion. For example, higher pressures can increase the density of molecules, leading to more frequent collisions and faster diffusion.
Self-diffusion is not limited to pure substances.
Self-diffusion can occur in mixtures, where different components exhibit their own diffusion rates based on their properties. This plays a crucial role in processes such as osmosis and the diffusion of solutes in biological systems.
Self-diffusion has important implications in materials science.
Understanding self-diffusion is crucial for developing and improving materials with desired properties. Diffusion rates determine how quickly substances can move through materials, affecting processes like heat conduction, mass transport, and the growth of crystals.
Self-diffusion can be measured experimentally.
Scientists use various techniques, such as diffusion cells and nuclear magnetic resonance (NMR) spectroscopy, to measure the rate and behavior of self-diffusion. These experiments provide valuable insights into the diffusion mechanisms of different substances.
Self-diffusion in liquids contributes to the concept of viscosity.
The flow of liquids is influenced by self-diffusion, with higher diffusion rates leading to lower viscosity. Viscosity is a measure of a liquid’s resistance to flow and plays a role in numerous applications, from lubrication to paint manufacturing.
Self-diffusion plays a role in drug delivery systems.
In pharmaceutical sciences, understanding the self-diffusion of drugs within the body is crucial for designing effective drug delivery systems. Diffusion properties influence how quickly and efficiently drugs can reach their target sites.
Self-diffusion is a dynamic process.
Particles are constantly in motion, resulting in continuous self-diffusion. This dynamic behavior is a fundamental characteristic of matter and is responsible for the mixing and distribution of substances over time.
Self-diffusion is affected by the presence of obstacles.
When particles encounter obstacles such as other molecules or imperfections in a solid lattice, their diffusion can be hindered or slowed down. This phenomenon is particularly relevant in materials engineering and the study of diffusion in porous solids.
Self-diffusion plays a role in chemical reactions.
In chemical reactions, self-diffusion allows reactant molecules to come into contact with each other, increasing the likelihood of successful collisions and reaction rates. Diffusion is critical for the progress of reactions in both gaseous and liquid phases.
Self-diffusion is a fundamental process in the universe.
From the diffusion of particles in interstellar space to the dispersion of pollutants in the atmosphere, self-diffusion occurs on a vast scale throughout the universe. Understanding the principles of self-diffusion contributes to our knowledge of the cosmos.
These 16 captivating facts about self-diffusion highlight the importance and wide-ranging applications of this fundamental process in science and everyday life. Whether it’s the migration of atoms in a solid or the transport of molecules in our bodies, self-diffusion plays a profound role in shaping the world around us.
Conclusion
In conclusion, self-diffusion is a fascinating phenomenon that occurs in various substances and plays a crucial role in many natural and industrial processes. Through self-diffusion, particles are able to move and mix on their own, leading to the distribution of substances and the equalization of concentration gradients.
We learned that self-diffusion is influenced by factors such as temperature, pressure, concentration, and molecular size. It follows Fick’s laws of diffusion and can be quantified using diffusion coefficients. Additionally, self-diffusion is not limited to just liquids, but also occurs in gases and solids.
Understanding the principles of self-diffusion is vital in fields like chemistry, physics, and materials science. It has numerous applications, ranging from understanding biological processes in cells to optimizing industrial processes like heat treatment and alloy formation.
Overall, self-diffusion opens up a world of possibilities for exploration and innovation. By delving deeper into this captivating phenomenon, scientists can continue to unlock new insights and advancements in various fields.
FAQs
Q: What is self-diffusion?
A: Self-diffusion refers to the movement of particles within a substance, where the particles themselves are responsible for the diffusion process.
Q: What factors influence self-diffusion?
A: Self-diffusion is influenced by factors such as temperature, pressure, concentration, and molecular size.
Q: How is self-diffusion quantified?
A: Self-diffusion can be quantified using diffusion coefficients, which describe the rate of diffusion for a specific substance under given conditions.
Q: Does self-diffusion occur in all states of matter?
A: Yes, self-diffusion occurs in gases, liquids, and solids, although the mechanisms may differ depending on the state of matter.
Q: What are some practical applications of self-diffusion?
A: Self-diffusion has various practical applications, such as understanding biological processes in cells, optimizing industrial processes like heat treatment and alloy formation, and creating efficient material designs.
Q: How does self-diffusion contribute to equalizing concentration gradients?
A: Through self-diffusion, particles move from areas of higher concentration to areas of lower concentration, ultimately equalizing concentration gradients and achieving equilibrium.
Self-diffusion's captivating nature leaves you yearning for more scientific exploration. Uncover statistical mechanics principles that shaped Ludwig Boltzmann's groundbreaking work. Dive into materials science facts that will surprise and inspire you. Expand your knowledge with astounding diffusion facts, from everyday occurrences to cutting-edge applications. Embark on a journey of discovery as you navigate these fascinating topics, each offering unique insights into the world around us. Whether you're a curious learner or a seasoned scientist, these articles promise to captivate your mind and ignite your passion for scientific understanding.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
