The study of chemistry encompasses a vast and fascinating world of theories and concepts. One such theory that stands out is the Bronsted-Lowry theory. Developed by Danish chemists Johannes Bronsted and Thomas Lowry in the early 20th century, this theory revolutionized our understanding of acid-base reactions. It goes beyond the traditional definition of acids as substances that release hydrogen ions (H+) and bases as substances that accept them. Instead, the Bronsted-Lowry theory views acids as proton donors and bases as proton acceptors.
In this article, we will delve into the extraordinary facts about the Bronsted-Lowry theory that make it both fundamental and intriguing. From its impact on chemical equations to its implications in everyday life, these facts highlight the significance of this theory in the world of chemistry. So, let’s embark on a journey through the remarkable aspects of the Bronsted-Lowry theory and uncover its secrets.
Key Takeaways:
- The Bronsted-Lowry Theory redefined acids and bases, allowing us to understand how they interact in different solutions, not just water. It also helped predict their strengths and laid the foundation for advancements in chemistry.
- This theory showed that water can be both an acid and a base, and it’s not just about protons. It’s widely used in organic chemistry and has led to many new discoveries in the field.
The Bronsted-Lowry Theory revolutionized our understanding of acid-base reactions.
Developed independently by Johannes Bronsted and Thomas Lowry in the early 20th century, this groundbreaking theory provided a new perspective on how acids and bases interact with each other. Unlike the Arrhenius Theory, which defined acids as substances that release hydrogen ions in water and bases as substances that release hydroxide ions, the Bronsted-Lowry Theory expanded the definition to include reactions that occur in non-aqueous solutions.
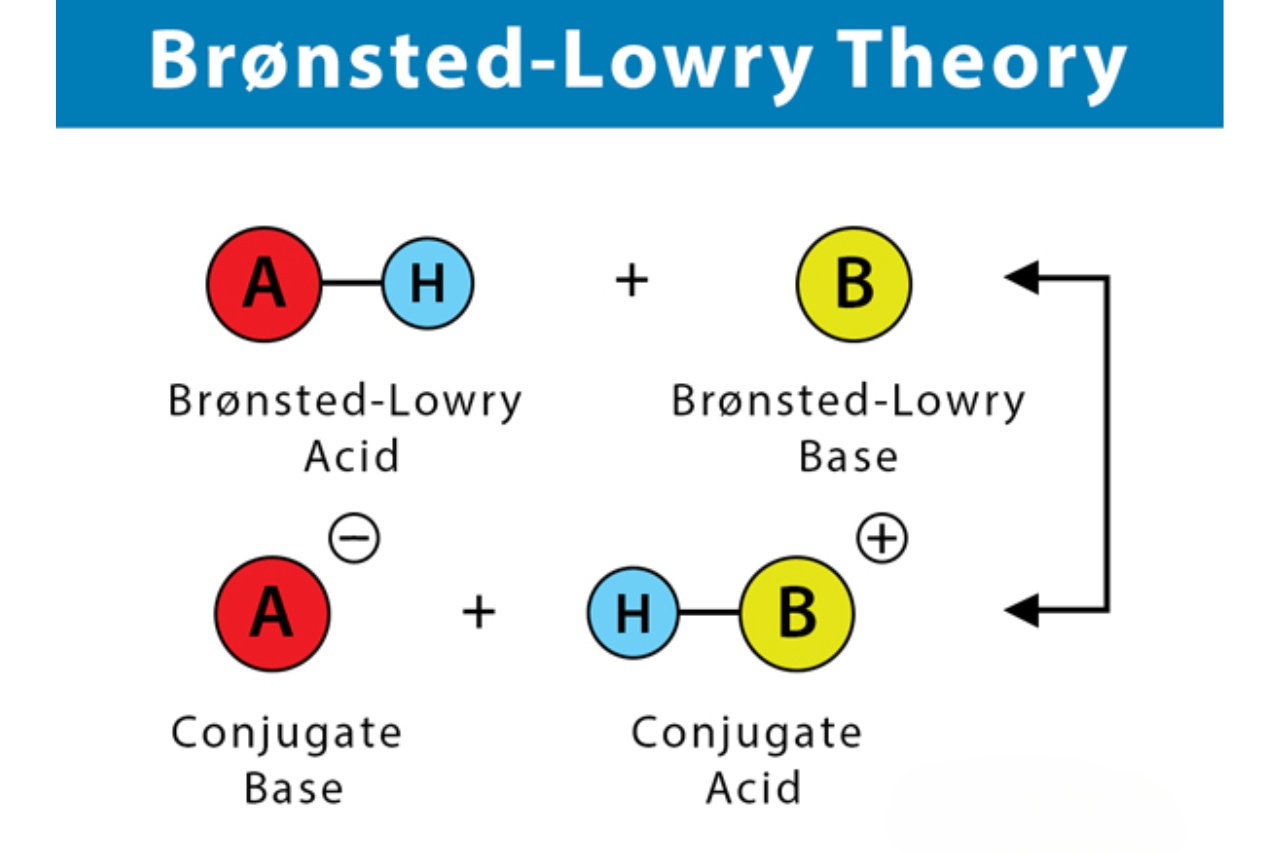
Acids are now defined as proton donors according to the Bronsted-Lowry Theory.
This expansion in definition allowed for a broader understanding of acid-base reactions. According to the theory, an acid is a species that donates a proton (H+) to another species, while a base is a species that accepts a proton.
The Bronsted-Lowry Theory provides a more comprehensive explanation of acid-base equilibrium.
Unlike the limited explanation provided by the Arrhenius Theory, the Bronsted-Lowry Theory takes into account both the forward and reverse reactions of acid-base equilibrium. This allows for a better understanding of how acids and bases behave in solution and how equilibrium is established.
Conjugate acid-base pairs play a crucial role in the Bronsted-Lowry Theory.
According to the theory, when an acid donates a proton, it forms its conjugate base, and when a base accepts a proton, it forms its conjugate acid. These pairs are essential in understanding the transfer of protons between species during acid-base reactions.
Water can act as both an acid and a base according to the Bronsted-Lowry Theory.
The Bronsted-Lowry Theory recognizes that water can donate a proton to a base, making it an acid, or accept a proton from an acid, making it a base. This dual nature of water has significant implications in various chemical reactions and biochemical processes.
The Bronsted-Lowry Theory is not limited to aqueous solutions.
Unlike the Arrhenius Theory, which focused solely on acid-base reactions in water, the Bronsted-Lowry Theory extends its applicability to non-aqueous solutions. This allows for a more comprehensive understanding of acid-base behavior in various contexts.
Acid-base reactions are not limited to proton transfer.
The Bronsted-Lowry Theory recognizes that acid-base reactions can involve more than just a transfer of protons. Complex reactions, such as Lewis acid-base reactions, involve the formation of coordinate covalent bonds between the acid and the base.
The Bronsted-Lowry Theory can explain the concept of amphoteric substances.
Amphoteric substances are those that can act as both acids and bases. According to the Bronsted-Lowry Theory, an amphoteric substance can either donate or accept protons, depending on the reaction conditions.
The Bronsted-Lowry Theory allows for the prediction of relative acid and base strengths.
By analyzing the stability of the conjugate base formed when an acid donates a proton, the Bronsted-Lowry Theory enables scientists to compare and predict the strength of different acids. Similarly, the stability of the conjugate acid formed when a base accepts a proton can determine the relative strength of bases.
The Bronsted-Lowry Theory is widely used in organic chemistry.
Organic chemists heavily rely on the Bronsted-Lowry Theory to understand and predict reaction mechanisms, identify acid or base catalysis, and design synthetic strategies. The theory’s versatility and application in various fields have solidified its importance in the realm of chemistry.
The Bronsted-Lowry Theory has laid the foundation for numerous advancements in chemistry.
Since its introduction, the Bronsted-Lowry Theory has paved the way for further research and discoveries in the field of acid-base reactions. It has provided a more comprehensive framework for understanding and studying chemical reactions, leading to advancements in various branches of chemistry.
Conclusion
In conclusion, the Bronsted-Lowry theory is a fundamental concept in chemistry that explains acid-base reactions based on proton transfer. It provides a comprehensive framework for understanding the behavior of acids and bases in various chemical reactions.
By recognizing that acids donate protons and bases accept protons, the Bronsted-Lowry theory allows scientists to predict and explain the outcomes of acid-base reactions. This theory has revolutionized the field of chemistry and has far-reaching applications in various industries, including pharmaceuticals, environmental science, and materials science.
Understanding the extraordinary facts about the Bronsted-Lowry theory, such as the concept of conjugate acid-base pairs and the role of solvents, enhances our comprehension of chemical reactions and their underlying principles. So, whether you’re a student or a professional in the field of chemistry, familiarizing yourself with this theory is essential for a deeper understanding of acid-base chemistry.
FAQs
1. What is the Bronsted-Lowry theory?
The Bronsted-Lowry theory is a concept in chemistry that explains acid-base reactions based on the transfer of protons. According to this theory, an acid is a substance that donates protons, while a base is a substance that accepts protons.
2. How does the Bronsted-Lowry theory differ from the Arrhenius theory?
The Arrhenius theory defines an acid as a substance that produces hydrogen ions (H+) in solution, while the Bronsted-Lowry theory defines an acid as a substance that donates protons. The Bronsted-Lowry theory is considered more comprehensive because it can explain acid-base reactions in solvents other than water.
3. What are conjugate acid-base pairs?
Conjugate acid-base pairs are related substances that differ only by the presence or absence of a proton. An acid and its corresponding base form a conjugate acid-base pair. For example, in the reaction between hydrochloric acid (HCl) and water (H2O), HCl donates a proton to water, forming the conjugate base chloride ion (Cl-) and the conjugate acid hydronium ion (H3O+).
4. What role do solvents play in acid-base reactions?
Solvents play a crucial role in acid-base reactions as they provide a medium for the transfer of protons. Different solvents can affect the strength and reactivity of acids and bases. For example, a strong acid in a polar solvent may ionize more readily than in a nonpolar solvent.
5. What are some practical applications of the Bronsted-Lowry theory?
The Bronsted-Lowry theory has a wide range of applications in various fields. It is used in pharmaceutical research to understand the behavior of drugs in the body, in environmental science to study pH levels in water bodies, and in materials science to design new catalysts and sensors.
The Bronsted-Lowry Theory's revolutionary insights into acid-base reactions and proton donors have paved the way for a deeper understanding of chemical processes. Curious minds can further explore the fascinating world of chemistry by delving into the intriguing properties of amphoteric substances, which exhibit both acidic and basic characteristics. Unraveling the enigmatic nature of these substances and their role in the broader context of acid-base reactions promises to be an enlightening journey for enthusiasts and experts alike.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
