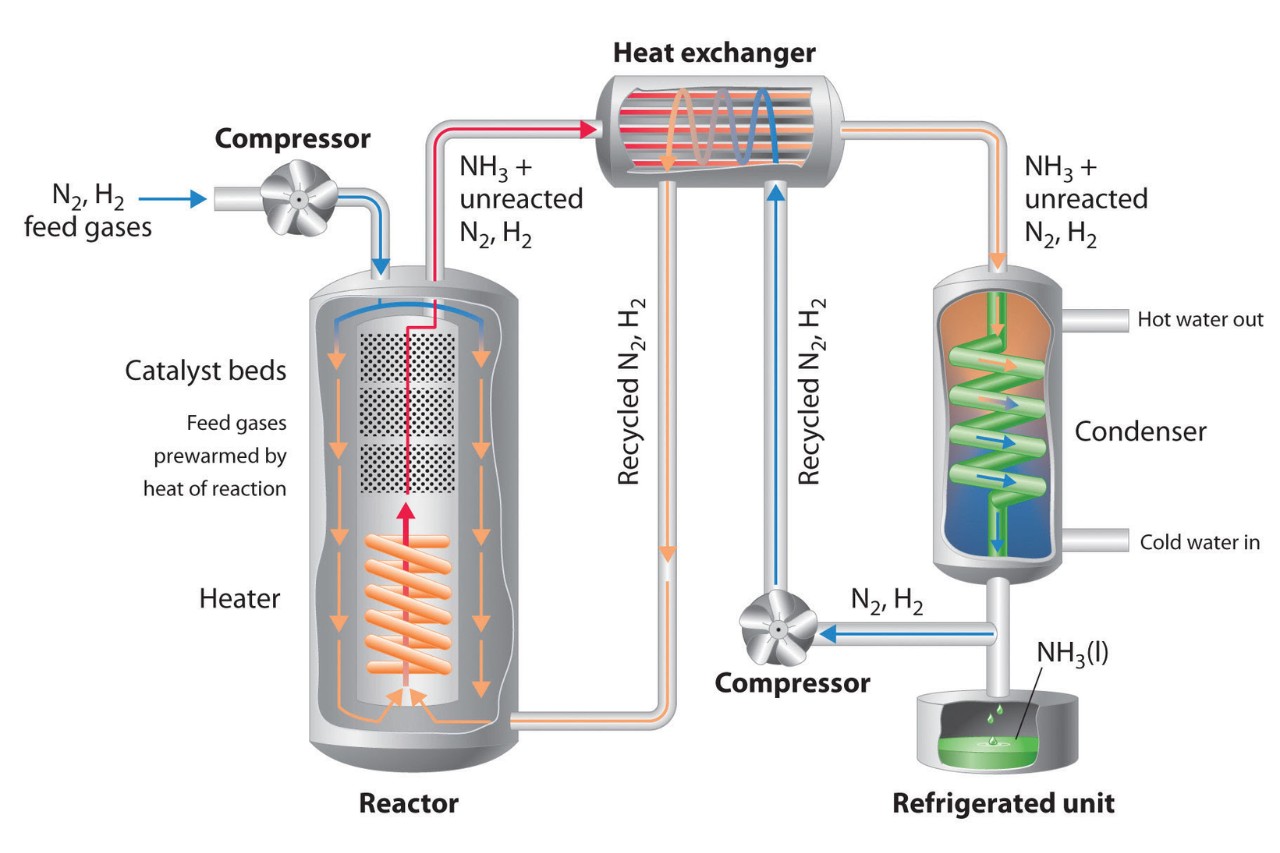
The Haber process is a groundbreaking chemical reaction that has had a profound impact on the world of chemistry and beyond. Developed by German chemist Fritz Haber in the early 20th century, this process revolutionized the production of ammonia, a vital component for the manufacturing of fertilizers, explosives, and other essential chemicals. The Haber process involves the synthesis of ammonia from nitrogen and hydrogen gases under specific temperature and pressure conditions.
In this article, we will explore 14 fascinating facts about the Haber process, shedding light on its significance, applications, challenges, and contributions to the world. From its historical origins to its environmental implications, this article will provide a comprehensive understanding of the Haber process and its impact on various industries and agricultural practices.
Key Takeaways:
- The Haber Process revolutionized agriculture and explosives by producing ammonia at high pressures and temperatures, impacting food production and global warfare.
- Despite its environmental challenges, the Haber Process continues to inspire innovation in chemistry, paving the way for sustainable industrial reactions and societal transformation.
The Haber Process enables the large-scale production of ammonia.
The Haber Process is a chemical reaction that converts nitrogen gas (N2) from the air and hydrogen gas (H2) derived from natural gas or methane (CH4) into ammonia (NH3). This reaction is essential for the production of fertilizers, as ammonia serves as a crucial ingredient.
Fritz Haber and Carl Bosch co-developed the Haber-Bosch Process.
Fritz Haber’s groundbreaking work on the Haber Process was complemented by Carl Bosch, a renowned chemical engineer. Together, they refined the process and developed the technology necessary for its large-scale implementation.
The Haber Process is a chemical equilibrium reaction.
The conversion of nitrogen gas and hydrogen gas into ammonia occurs through a reversible reaction. Maintaining an optimal balance of reactants, pressure, and temperature is crucial for achieving maximum ammonia production.
Iron catalysts play a vital role in the Haber Process.
The reaction is facilitated by the use of iron catalysts, which enhance the conversion of nitrogen and hydrogen and increase the overall efficiency of the process. Other catalysts, such as osmium or uranium, have also been studied for this purpose.
The Haber Process operates at high pressures.
To maximize ammonia production, the Haber Process typically operates at pressures ranging from 150 to 200 atmospheres. These high pressures help overcome the thermodynamic limitations of the reaction.
The reaction temperature affects the Haber Process.
While higher temperatures can expedite the reaction kinetics, they also reduce the equilibrium yield of ammonia. Therefore, the process is usually operated at temperatures between 400 to 450 degrees Celsius to achieve a balance between reaction rate and ammonia production.
The Haber Process was crucial for the production of explosives during World War I.
During World War I, the Haber Process played a pivotal role in the arms manufacturing industry by enabling the large-scale production of explosives, including ammonium nitrate.
The Haber Process has transformed global agriculture.
Thanks to the Haber Process, the production of nitrogen-based fertilizers has become economically viable on a massive scale. This has revolutionized modern agriculture, increasing crop yields and contributing to the global food supply.
The Haber Process has both positive and negative environmental impacts.
While the process has significantly contributed to food production, it also generates greenhouse gases, such as carbon dioxide. The management of these emissions remains an ongoing challenge.
The Haber Process is an energy-intensive process.
Large-scale ammonia production requires substantial amounts of energy. Advances in energy-efficient technologies continue to be explored to minimize the environmental footprint of the process.
The Haber Process is continuously optimized.
Scientists and engineers are continually researching ways to enhance the efficiency of the process. Improving catalysts, exploring alternative feedstocks, and optimizing reaction conditions are just a few areas of ongoing innovation.
The Haber Process paved the way for other industrial chemical reactions.
The success of the Haber Process has inspired the development of similar chemistries to produce other important substances, such as synthetic fuels and polymers.
The Haber Process is essential for the synthesis of numerous chemical compounds.
In addition to ammonia, the Haber Process enables the synthesis of various chemical compounds, including nitric acid, urea, and hydrogen cyanide, among others, which are pivotal in many industries.
The Haber Process has had a lasting impact on society.
The Haber Process has transformed the agricultural and chemical industries, shaping modern society’s ability to sustain food production and meet the demands of a growing population. Its scientific principles and technological advancements continue to be studied and appreciated.
In Conclusion
The 14 fascinating facts about the Haber Process shed light on its historical significance, scientific principles, and wide-ranging impacts. This revolutionary process has not only transformed agriculture and explosives manufacturing but also paved the way for numerous industrial chemical reactions. As researchers and engineers continue to optimize the process and explore sustainable alternatives, the Haber Process remains a testament to the power of chemistry in shaping our world.
Conclusion
In conclusion, the Haber Process is an incredible chemical reaction that has revolutionized the production of ammonia and played a vital role in the development of modern agriculture and industry. The process, which involves combining nitrogen and hydrogen gases over a catalyst at high temperatures and pressures, allows for the mass production of ammonia, which is then used to create fertilizers, explosives, and various other chemical products.Through the Haber Process, Fritz Haber and Carl Bosch made significant contributions to the field of chemistry, earning them the Nobel Prize in Chemistry in 1918. Their work not only had a profound impact on agriculture and industry but also played a crucial role in the growth of the global population by ensuring an abundant food supply.The Haber Process continues to be widely used today, driving the production of ammonia on a massive scale. It remains a fascinating area of study for chemists and serves as a testament to the incredible advancements that can be made through scientific research and discovery.
FAQs
Q: What is the Haber Process?
A: The Haber Process is a chemical reaction that synthesizes ammonia from nitrogen and hydrogen gases.
Q: Who developed the Haber Process?
A: The Haber Process was developed by Fritz Haber and Carl Bosch in the early 20th century.
Q: Why is the Haber Process important?
A: The Haber Process is important because it allows for the mass production of ammonia, which is used in the creation of fertilizers, explosives, and various chemical products.
Q: What are the conditions required for the Haber Process?
A: The Haber Process requires high temperatures, typically around 450-550 degrees Celsius, and high pressures, usually around 200-300 atmospheres.
Q: What is the significance of the Haber Process in agriculture?
A: The Haber Process revolutionized agriculture by making it possible to produce synthetic fertilizers on a large scale, which greatly increased crop yields and helped feed a growing global population.
Q: Is the Haber Process still used today?
A: Yes, the Haber Process is still widely used today for the production of ammonia, which has numerous industrial applications.
If you're fascinated by the Haber Process, don't miss our other captivating articles. Discover the world of chemical engineering and its countless applications. Explore the intricacies of industrial chemistry, including the Ostwald Process. Dive into the crucial role of nitrogen fixation in our ecosystem and agriculture. From the lab to the factory floor, uncover the science behind the processes that shape our modern world. Join us on this journey of discovery and expand your knowledge of chemistry and its real-world impact.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
