Ionization is a fascinating concept in the world of chemistry that plays a crucial role in various processes and phenomena. Understanding ionization is essential for grasping the behavior of atoms and molecules, as well as how they interact with each other and their surroundings. In simple terms, ionization refers to the process of gaining or losing electrons, resulting in the formation of ions. These ions, with their positive or negative charge, have unique properties and behaviors that distinguish them from neutral atoms and molecules. In this article, we will explore nine extraordinary facts about ionization that will not only expand your knowledge but also leave you in awe of the intricate world of chemistry.
Key Takeaways:
- Ionization is the process of atoms gaining or losing electrons, creating charged ions. It’s crucial in chemistry, technology, and even affects the colors of flames. It’s like atoms playing a game of “electronic hot potato”!
- Ionization also has a big impact on our world, from the colors of flames to the conduction of electricity. It’s like the secret ingredient that makes chemistry, technology, and even the atmosphere work!
What is Ionization?
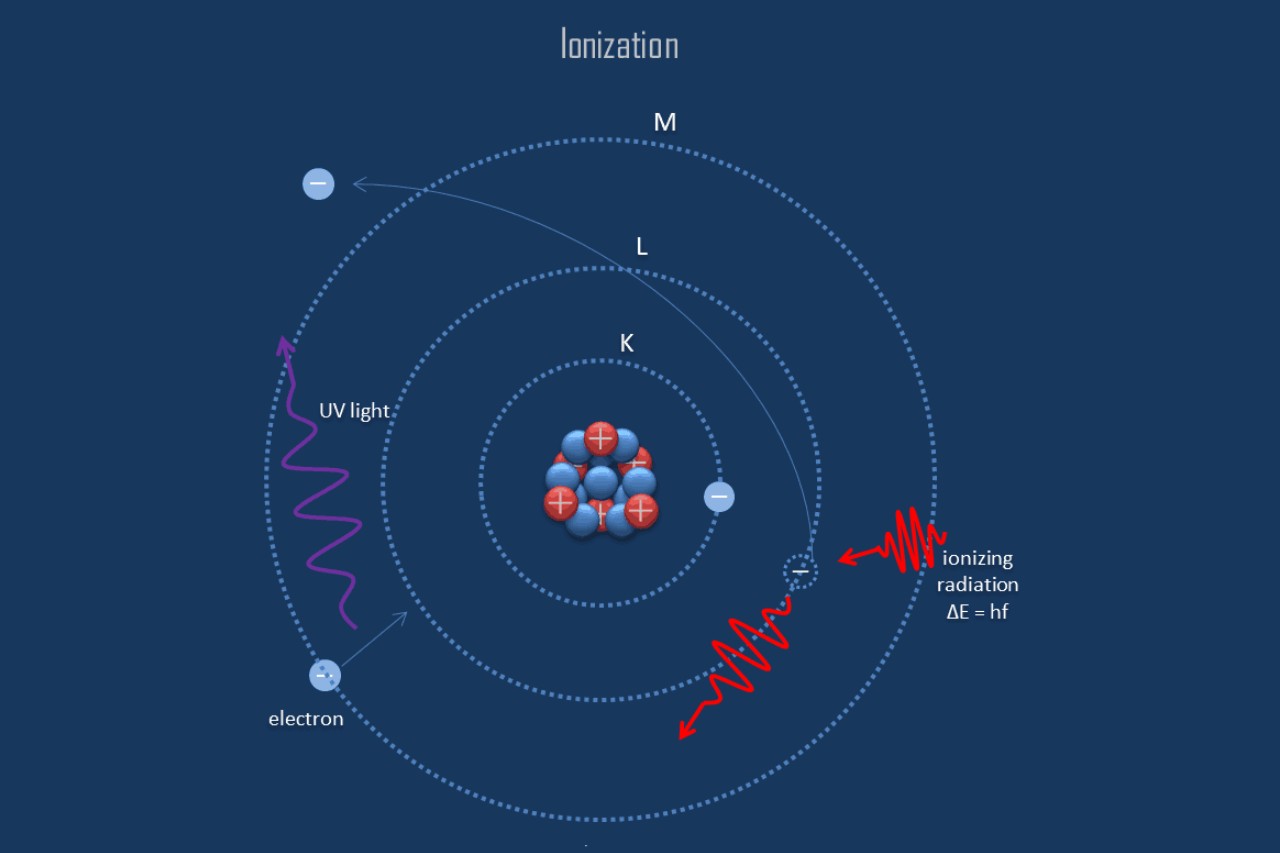
Ionization is the process by which an atom or molecule gains or loses electrons, resulting in the formation of charged particles called ions. It occurs when an atom or molecule is exposed to an energy source, such as heat, light, or electricity.
Role in Chemistry
Ionization plays a crucial role in chemistry as it is involved in various chemical reactions and processes. It enables the formation of new compounds and facilitates the transfer of electrons between atoms, leading to the creation of stable molecules.
Common Forms of Ionization
There are several common forms of ionization, including photoionization, collisional ionization, and electron impact ionization. Photoionization occurs when an atom or molecule absorbs photons, while collisional ionization involves collisions with other particles. Electron impact ionization occurs when high-energy electrons collide with atoms or molecules.
Applications in Technology
Ionization has numerous applications in technology. It is utilized in mass spectrometry, a technique used to identify and analyze chemical compounds. Ionization is also essential in plasma technology, which is employed in various fields such as material processing, surface modification, and energy production.
Atmospheric Ionization
In the Earth’s atmosphere, ionization occurs naturally due to cosmic rays, ultraviolet radiation from the sun, and lightning. These processes lead to the formation of ions, which play a vital role in the chemistry and dynamics of the atmosphere.
Biological Effects of Ionization
Ionizing radiation, such as X-rays and gamma rays, can have biological effects on living organisms. It can cause DNA damage and increase the risk of cancer. However, it is also utilized in medical imaging and radiation therapy to diagnose and treat diseases.
Ionization Energy
Ionization energy is the energy required to remove an electron from an atom or molecule. It varies depending on the element and its electron configuration. Elements with low ionization energy are more likely to form ions compared to those with high ionization energy.
Ionization and Flame Colors
Ionization plays a role in the different colors observed in flames. When atoms are heated in a flame, their electrons absorb energy and move to higher energy levels. As the electrons return to their original levels, they release energy in the form of light, resulting in the characteristic colors of the flame.
Ionization and Electric Current
Ionization is essential for the conduction of electric current in various materials. When an electric field is applied, ionization allows the flow of charged particles, enabling the transmission of electricity through conductors.
Conclusion
Ionization is a fascinating process that plays a crucial role in various aspects of our lives. From the formation of ions in chemical reactions to the ionization of gases in the Earth’s atmosphere, this phenomenon has a profound impact on our understanding of the world.
By understanding the extraordinary facts about ionization, we gain insights into the behavior of matter at the atomic and molecular level. Ionization enables the creation of charged particles that can be harnessed for numerous applications, such as in batteries, fuel cells, and even medical treatments.
As we continue to explore the intricacies of ionization, we unlock new possibilities for technological advancements and scientific discoveries. The study of ionization will undoubtedly continue to shape our future, pushing the boundaries of what is possible in the realm of chemistry and beyond.
FAQs
Q: What is ionization?
A: Ionization is the process by which an atom or molecule gains or loses electrons, resulting in the formation of ions.
Q: What causes ionization?
A: Ionization can be caused by various factors, such as exposure to high-energy radiation, collision with other particles, or the application of an electric field.
Q: Why is ionization important?
A: Ionization is important because it leads to the creation of charged particles, which play a vital role in chemical reactions, electricity conduction, and atmospheric processes.
Q: How is ionization used in everyday life?
A: Ionization has numerous practical applications, including the operation of batteries, the production of electricity in fuel cells, and the sterilization of air and water in medical and industrial settings.
Q: Can ionization be harmful?
A: Yes, depending on the circumstances. Ionizing radiation, such as X-rays or radioactive materials, can be harmful to living organisms and should be handled with caution.
Q: How does ionization affect the Earth’s atmosphere?
A: Ionization is responsible for the formation of the ionosphere, a region in the Earth’s upper atmosphere that influences radio wave propagation and plays a role in the Earth’s climate system.
Q: Can ionization be reversed?
A: Yes, ionization can be reversed through processes such as recombination, where ions recombine with free electrons to form neutral atoms or molecules.
Q: Are ions only found in gases?
A: No, ions can be found in various states of matter, including gases, liquids, and solids. However, the mobility of ions is usually higher in gases and liquids compared to solids.
Q: How is ionization studied?
A: Ionization is studied through experimental techniques such as mass spectrometry, ion chromatography, and spectroscopy, as well as theoretical models and simulations.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
