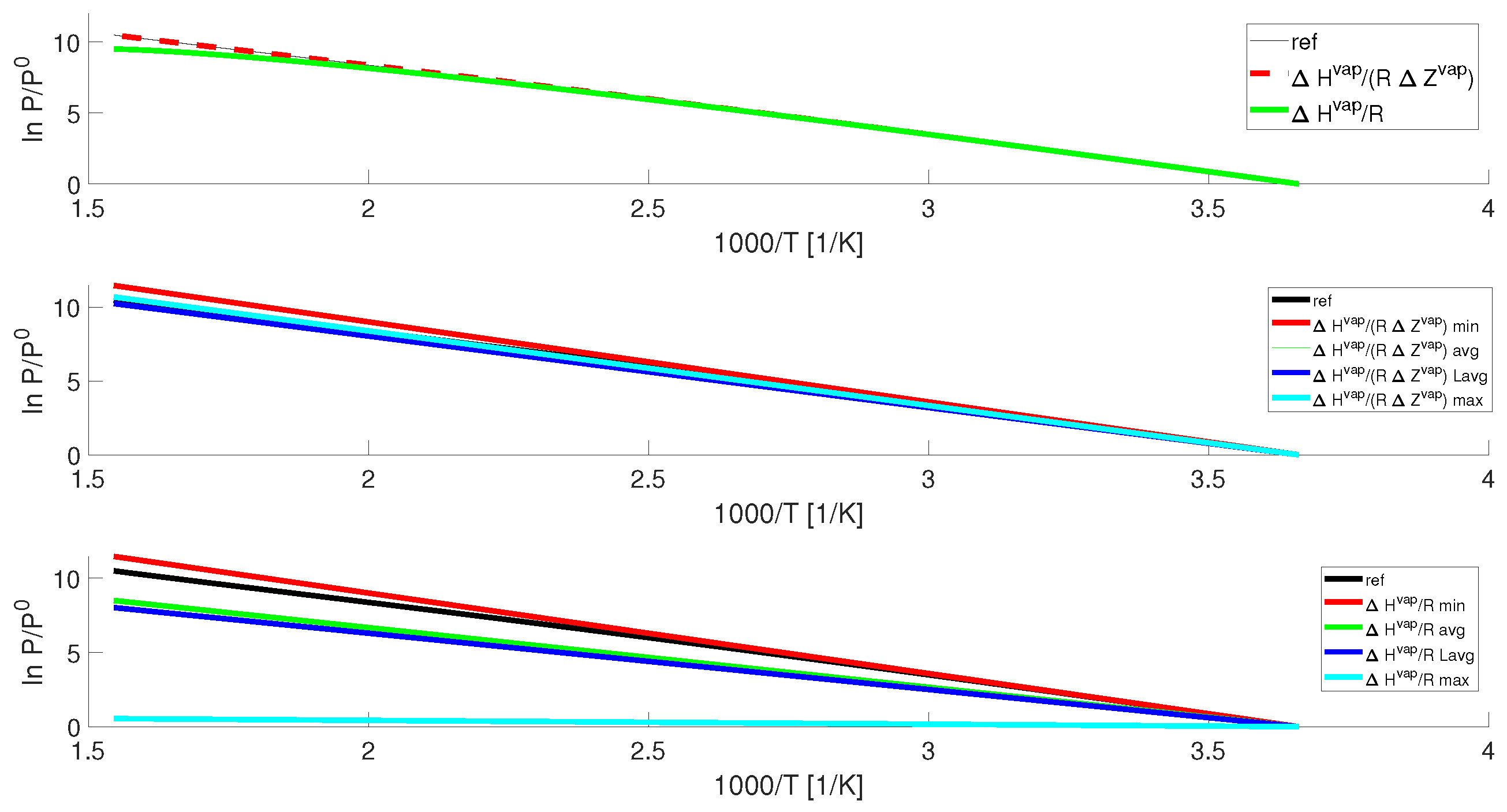
The Clausius-Clapeyron equation is a fundamental concept in the field of thermodynamics and holds great significance in understanding the behavior of gases and vapors. Developed in the 19th century by Rudolf Clausius and Benoît Paul Émile Clapeyron, this equation provides insights into the relationship between temperature, pressure, and the phase equilibrium of substances.
In this article, we will uncover 14 mind-blowing facts about the Clausius-Clapeyron equation that will deepen your understanding of its applications and implications in the world of chemistry. From its origins to its practical uses, prepare to be amazed by the intricacies and versatility of this equation.
Key Takeaways:
- The Clausius-Clapeyron Equation is a powerful tool that helps us understand how temperature and pressure affect phase transitions, from boiling to cloud formation in meteorology.
- This equation, developed by Clausius and Clapeyron, is crucial in studying thermodynamics, phase diagrams, and even optimizing industrial processes. Its applications span across various scientific fields, making it a fundamental concept to grasp.
The Clausius-Clapeyron Equation is a fundamental equation in thermodynamics.
The Clausius-Clapeyron Equation is a powerful tool used to describe the relationship between temperature and vapour pressure during phase transitions. It helps us understand how the equilibrium vapour pressure of a substance changes with temperature.
It was developed by Rudolf Clausius and Benoit Paul Emile Clapeyron.
The Clausius-Clapeyron Equation is named after its two pioneers, Rudolf Clausius and Benoit Paul Emile Clapeyron. They independently derived this equation in the mid-19th century, revolutionizing the field of thermodynamics.
It applies to phase transitions between two states of matter.
The Clausius-Clapeyron Equation is applicable to phase transitions such as vaporization, condensation, melting, and sublimation. It provides insights into how the equilibrium conditions change with temperature and pressure during these transitions.
The equation can predict the boiling point of a substance at different pressures.
By using the Clausius-Clapeyron Equation, we can estimate the boiling point of a substance at different pressures. This is particularly useful for understanding how changes in pressure affect the boiling point of liquids.
It relies on the concept of entropy.
The Clausius-Clapeyron Equation is based on the concept of entropy, which is a measure of the disorder in a system. The equation considers how changes in entropy influence the phase transition and the equilibrium conditions.
The equation is applicable to ideal gases and liquids.
While the Clausius-Clapeyron Equation can be applied to both ideal gases and liquids, it is most accurate for substances that closely follow ideal gas behavior. For liquids, it assumes that the heat of vaporization remains constant over the temperature range of interest.
It has applications in meteorology and atmospheric science.
The Clausius-Clapeyron Equation plays a crucial role in meteorology and atmospheric science. It helps us understand how changes in temperature and pressure affect the formation of clouds, the release of moisture, and the occurrence of precipitation.
The equation can be used to study phase diagrams.
Phase diagrams illustrate the different phases of a substance under varying temperature and pressure conditions. The Clausius-Clapeyron Equation is instrumental in constructing and analyzing phase diagrams by providing insights into the equilibrium conditions at different points.
It is derived from the laws of thermodynamics.
The Clausius-Clapeyron Equation is derived from the first and second laws of thermodynamics, which govern the principles of energy conservation and entropy. It is a mathematical representation of these laws and their implications for phase transitions.
The equation assumes an idealized, constant enthalpy process.
To simplify calculations, the Clausius-Clapeyron Equation assumes that the process occurring during a phase transition is an idealized, constant enthalpy process. This assumption allows for a simpler mathematical formulation.
It is applicable to reversible phase transitions.
The Clausius-Clapeyron Equation applies to reversible phase transitions, where the system can go back and forth between the two states without any hysteresis or energy losses. It provides a theoretical framework for understanding such transitions.
Temperature and pressure affect the phase transition rate.
According to the Clausius-Clapeyron Equation, temperature and pressure have a significant impact on the rate of phase transitions. Changes in these parameters can either accelerate or decelerate the transition, leading to noticeable effects on the system.
The equation is a cornerstone in the study of thermodynamics.
The Clausius-Clapeyron Equation is considered a cornerstone in the study of thermodynamics. It provides a quantitative description of the relationship between temperature, pressure, and phase transitions, enabling scientists to analyze and predict the behavior of complex systems.
It can be applied to various fields of science and engineering.
Due to its versatility and broad applicability, the Clausius-Clapeyron Equation finds uses in numerous fields of science and engineering. It aids in the design and optimization of industrial processes, the study of materials and their phase behavior, and the analysis of climate and weather phenomena.
As we have seen, the 14 Mind-blowing Facts About Clausius-Clapeyron Equation highlight its significance and applications in the realms of thermodynamics, phase transitions, atmospheric science, and beyond. Understanding this equation allows us to unravel the complexities of temperature, pressure, and equilibrium conditions during phase changes, leading to remarkable insights and advancements in various scientific disciplines.
Conclusion
In conclusion, the Clausius-Clapeyron equation is a fundamental and powerful tool in thermodynamics and atmospheric science. Its application extends to a wide range of fields, from chemistry to meteorology, allowing scientists and engineers to predict and understand the behavior of different substances and systems.Through the equation, we can explore the relationship between temperature, pressure, and the vaporization or condensation of a substance. It provides valuable insights into phase transitions and can be used to calculate parameters such as enthalpy, entropy, and vapor pressure. The equation’s simplicity and versatility make it an essential tool for researchers and practitioners in various domains.By understanding the underlying principles of the Clausius-Clapeyron equation, we can gain a deeper insight into the thermodynamic properties of substances and their behavior under different conditions. It serves as a foundation for more advanced theories and models, enabling us to explore complex phenomena and make predictions with confidence.Overall, the Clausius-Clapeyron equation is a fascinating topic that showcases the beauty and power of chemistry and thermodynamics. Exploring its applications and implications opens up a world of possibilities for continued scientific discovery and technological advancements.
FAQs
1. What is the Clausius-Clapeyron equation?
The Clausius-Clapeyron equation is an equation used in thermodynamics to describe the relationship between temperature, pressure, and the phase transition of a substance.
2. How is the Clausius-Clapeyron equation derived?
The equation is derived from the principles of thermodynamics and statistical mechanics, combining concepts such as the ideal gas law and the laws of phase transitions.
3. What are the applications of the Clausius-Clapeyron equation?
The equation is widely used in various fields, including chemistry, meteorology, and materials science, to predict and understand phase transitions, vapor pressure, and other thermodynamic properties.
4. Can the Clausius-Clapeyron equation be applied to all substances?
The equation is generally applicable to substances that undergo phase transitions, such as vaporization or condensation. However, it may not be accurate for all substances, especially those with complex molecular structures or non-ideal behavior.
5. Are there any limitations to using the Clausius-Clapeyron equation?
While the equation is a valuable tool, it assumes several simplifications and ideal conditions. In practice, factors such as non-ideal behavior, intermolecular forces, and external influences may affect the accuracy of predictions made using the equation.
The Clausius-Clapeyron equation's fascinating insights into thermodynamics and phase transitions are just the beginning of your scientific journey. Dive deeper into the captivating world of equilibrium and uncover its secrets. Explore the crucial role of environmental science in protecting our planet's delicate balance. Immerse yourself in the thrilling field of meteorology, where weather watching becomes an adventure. Embark on a quest for knowledge that will expand your horizons and ignite your passion for science.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


