The Lever Rule may sound like a mysterious concept for those not familiar with the world of chemistry, but it is actually a fundamental principle that plays a crucial role in determining the composition of mixtures. Understanding the Lever Rule can provide valuable insights into the behavior of solutions and help scientists make predictions about phase changes and equilibrium conditions.
In this article, we will delve into the fascinating world of the Lever Rule and explore 12 enigmatic facts that will shed light on its importance and application in the field of chemistry. From its origins to its practical uses, we will uncover the hidden secrets behind this powerful tool and uncover how it unlocks the mysteries of mixtures.
Key Takeaways:
- The Lever Rule helps us understand how much of each material is present in a mixture, like figuring out the ingredients in a recipe. It’s crucial for making strong and useful materials in industries.
- By using the Lever Rule, scientists and engineers can design new materials with special properties, like making a super-strong metal for a spaceship or a super-flexible material for a new phone.
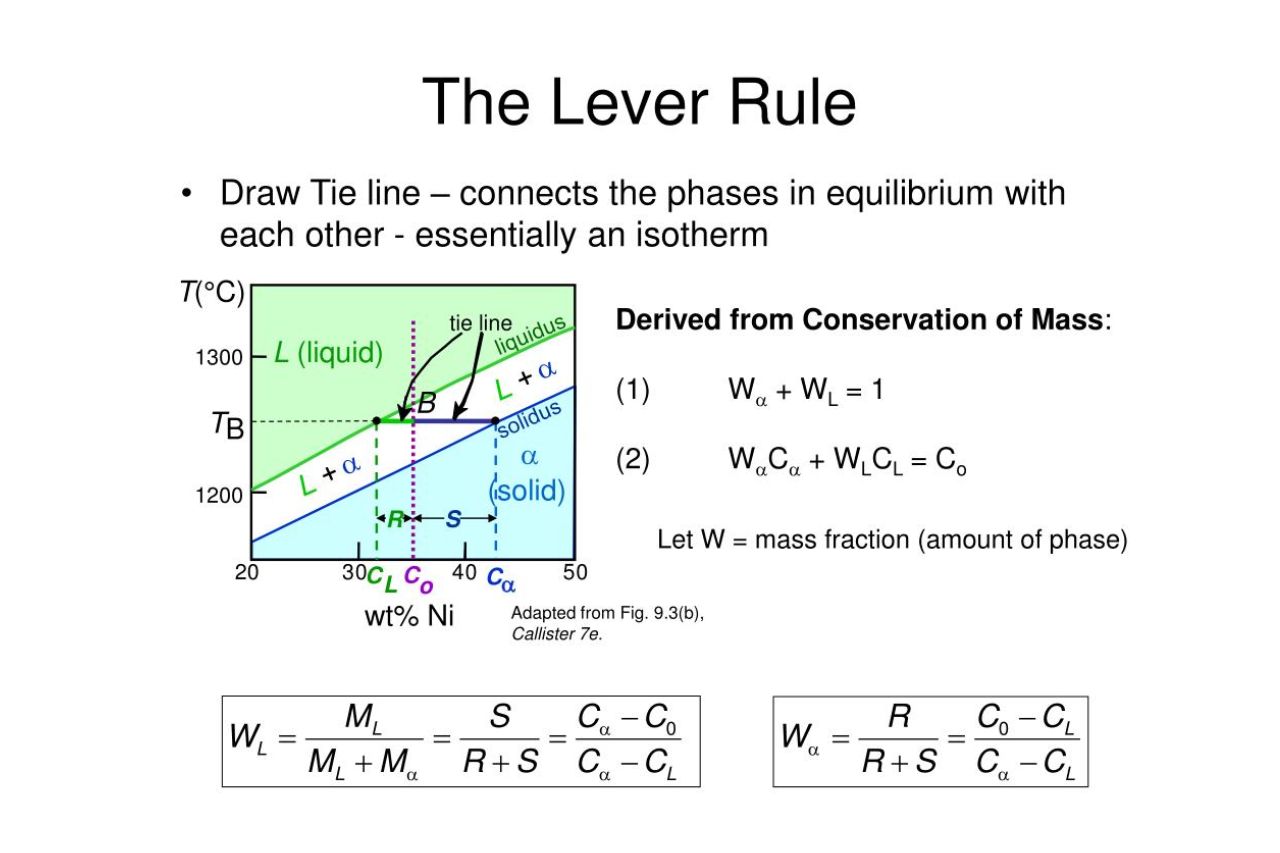
The Lever Rule and its Significance
The Lever Rule is a fundamental principle used to determine the proportion of phases present in a binary alloy system. It plays a vital role in understanding phase transformations, such as solidification and phase separation, which are crucial in numerous industrial applications.
Origin of the Lever Rule
The Lever Rule was named after its resemblance to a lever in mechanics. Just like a lever helps in finding balance, the Lever Rule enables us to find the balance of phases in a materials system. It was first introduced by W. H. Lever in 1869, hence the name Lever Rule.
The Lever Rule Equation
The Lever Rule is mathematically expressed as W1/W2 = L1/L2, where W1 and W2 represent the weight fractions of the two phases, and L1 and L2 represent the lengths of the tie-line intersecting the phase diagram. This equation allows us to quantitatively determine the proportions of different phases.
Applying the Lever Rule
By applying the Lever Rule, we can determine the weight fraction of each phase present in a binary alloy system. This information is essential for understanding material properties, such as strength, hardness, and conductivity.
Lever Rule and Microstructure Control
The Lever Rule has significant implications when it comes to controlling the microstructure of materials. By manipulating the composition and processing parameters, engineers can utilize the Lever Rule to achieve desired material properties with enhanced performance.
Lever Rule and Phase Diagrams
The Lever Rule is intricately linked to phase diagrams, which graphically represent the relationships between different phases in a materials system. Understanding the Lever Rule helps us interpret phase diagrams more effectively and provides insights into the behavior of materials under different conditions.
Lever Rule and Solidification
During solidification, when a liquid transforms into a solid, the Lever Rule enables us to determine the proportions of the solid phase and the remaining liquid phase. This information is crucial for controlling the solidification process and obtaining materials with desired properties.
Lever Rule and Eutectic Systems
In eutectic systems, where two or more phases coexist at a specific composition and temperature, the Lever Rule helps in determining the weight fractions of each phase. This knowledge is essential for optimizing materials for specific applications, such as alloys used in aerospace or automotive industries.
Lever Rule and Phase Separation
Phase separation occurs when two immiscible phases separate from a homogeneous mixture. The Lever Rule enables us to quantify the proportions of each phase during phase separation, leading to a better understanding of phenomena like spinodal decomposition and domain formation.
Lever Rule and Material Characterization
Materials characterization techniques, such as microscopy and spectroscopy, can be combined with the Lever Rule to analyze and identify the phases present in a material. This allows scientists and engineers to gain valuable insights into the structure and properties of materials.
Limitations of the Lever Rule
Although the Lever Rule is a powerful tool, it does have its limitations. It assumes ideal conditions, such as complete thermodynamic equilibrium and uniform composition, which may not always be applicable in real-world scenarios.
Lever Rule and Material Design
The Lever Rule plays a crucial role in material design and optimization. By understanding the Lever Rule and its implications on phase proportions, engineers can develop innovative materials with tailored properties to meet specific performance requirements.
So, there you have it – 12 enigmatic facts about Lever Rule that have shed light on its significance and applications in the world of materials science. Whether you’re a student, researcher, or simply curious about the fascinating world of alloys and phase transformations, understanding the Lever Rule is a fundamental step towards unraveling the mysteries of material behavior. Unlock the potential of Lever Rule, master the phase diagrams, and embark on a journey of discovery!
Conclusion
The Lever Rule is a fundamental concept in the field of chemistry that describes the relationship between the composition and phases of a substance in equilibrium. Understanding how to apply the Lever Rule can provide valuable insights into the behavior of mixtures and alloys, enabling scientists and engineers to make informed decisions in various industries.
By effectively utilizing the Lever Rule, researchers can predict the proportions of different phases in a mixture, determine the conditions necessary to achieve desired compositions, and optimize material properties. This powerful tool allows for the manipulation and control of the structural and chemical properties of substances, bringing significant advancements in fields such as metallurgy, material science, and chemical engineering.
Exploring the enigmatic facts about the Lever Rule provides a deeper understanding of its importance and impact in the scientific community. As we continue to unravel the mysteries of this rule, we unlock new possibilities and pave the way for exciting discoveries and innovations in the world of chemistry.
FAQs
Q: What is the Lever Rule?
A: The Lever Rule is a principle in chemistry that relates the compositions and amounts of different phases in a substance at equilibrium.
Q: How is the Lever Rule used in practical applications?
A: The Lever Rule is used in various industries to determine the proportions of different phases in mixtures, optimize material properties, and make informed decisions about composition and structure.
Q: Can the Lever Rule be applied to all types of mixtures?
A: Yes, the Lever Rule is applicable to mixtures of solid phases, liquid phases, and combinations thereof.
Q: Are there any limitations to the Lever Rule?
A: While the Lever Rule provides valuable insights, it assumes ideal conditions and may not accurately predict phase behavior in complex systems or under extreme conditions.
Q: Can the Lever Rule be used to analyze phase changes during reactions?
A: Yes, the Lever Rule can be applied to analyze phase changes during reactions, providing a framework for understanding the equilibrium composition of the system.
Unraveling the mysteries of the Lever Rule is just the beginning. Dive deeper into <phase diagrams>, uncovering their intriguing secrets. Surprising facts about <materials science> await your discovery, revealing the hidden wonders of this fascinating field. For those captivated by the art and science of metals, extraordinary <metallurgy> facts will ignite your curiosity and expand your knowledge. Embark on a journey of exploration and let these enigmatic realms of science captivate your imagination.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
