Amphoterism is an intriguing concept in the world of chemistry. It refers to the ability of certain substances to exhibit both acidic and basic properties, depending on the conditions under which they are placed. This unique characteristic allows these substances to interact with both acids and bases, making them versatile and essential in various chemical reactions. In this article, we will explore 19 intriguing facts about amphoterism that highlight its significance and impact in the field of chemistry. From the discovery of amphoteric compounds to their role in buffering systems, we delve into the fascinating world of amphoterism and its applications. So, grab your lab coat and goggles, as we embark on a journey to uncover the secrets of this remarkable chemical phenomenon.
Key Takeaways:
- Amphoterism allows certain substances to act as both acids and bases, like water and metal oxides, impacting industries and biological processes.
- Understanding amphoterism opens up a world of possibilities in chemistry, from designing buffer solutions to treating acid burns and developing corrosion-resistant materials.
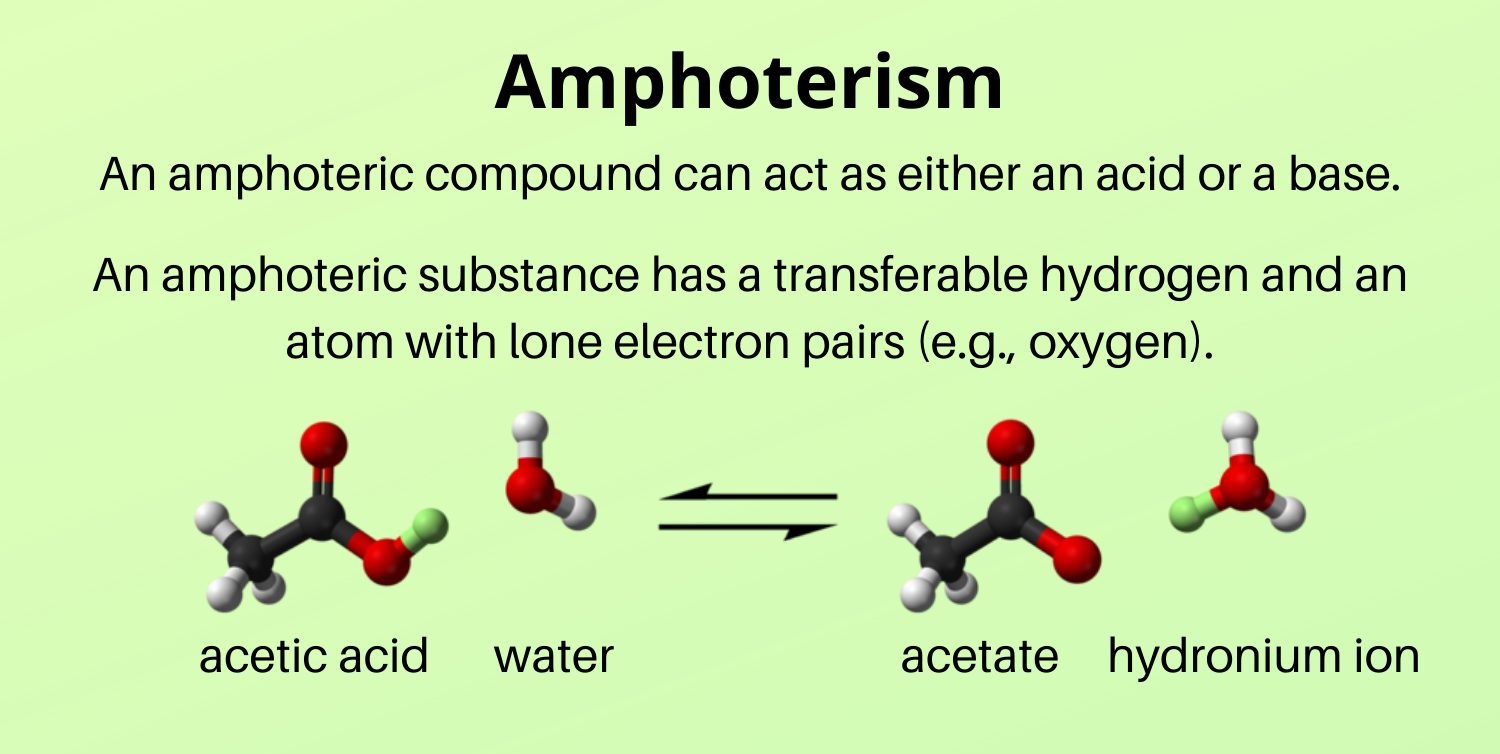
Amphoterism is the ability of a substance to act as both an acid and a base.
Amphoterism is a fascinating property exhibited by certain compounds that allows them to react with both strong acids and strong bases. This unique characteristic is what sets amphoterism apart from other chemical properties.
Water is one of the most well-known examples of an amphiprotic substance.
Water, H2O, is a prime example of amphoterism. It can act as both an acid and a base, depending on the reaction conditions. In an acidic solution, water can accept a proton, behaving as a base. Conversely, in a basic solution, water can donate a proton, acting as an acid.
Amphoterism is often observed in metal oxides.
Many metal oxides exhibit amphoterism, including zinc oxide (ZnO) and aluminum oxide (Al2O3). These compounds can react with both acids and bases, leading to a wide range of applications in various industries.
Amphoterism plays a crucial role in maintaining the pH balance in living organisms.
The ability of certain biomolecules to act as both acids and bases is vital for maintaining the pH balance in living organisms. This ensures optimal conditions for the functioning of biological processes.
Amphoterism can be observed in amino acids.
Amino acids, the building blocks of proteins, exhibit amphoterism. They contain both an amino group that can act as a base and a carboxyl group that can act as an acid. This property is crucial for the formation of peptide bonds during protein synthesis.
Zinc hydroxide is an example of an amphiprotic compound.
Zinc hydroxide, Zn(OH)2, is a compound that demonstrates amphoterism. It can react with both acids and bases, making it useful in various industrial applications, such as in the manufacturing of paints and coatings.
Amphoterism can be observed in certain minerals.
Minerals like amphoteric aluminum hydroxide (Al(OH)3) and amphoteric zinc carbonate (ZnCO3) exhibit amphoterism. This property makes them valuable in the pharmaceutical and cosmetic industries.
The concept of amphoterism was first proposed by Gilbert N. Lewis in 1923.
American chemist Gilbert N. Lewis introduced the concept of amphoterism in His groundbreaking work laid the foundation for further research and understanding of this unique chemical property.
Amphoterism is important in the design of buffer solutions.
Buffer solutions are crucial in various scientific and industrial processes. The amphiprotic nature of certain compounds allows them to maintain a stable pH in these solutions, preventing drastic changes in acidity or alkalinity.
Amphoterism can be observed in the behavior of certain metal ions.
Metal ions such as aluminum ions (Al3+) and zinc ions (Zn2+) exhibit amphoterism. They can react with both acids and bases, leading to complex chemical reactions and the formation of coordination compounds.
Amphoterism is utilized in the production of ceramics.
The amphiprotic nature of certain ceramics allows them to withstand extreme temperatures and resist chemical degradation. This makes them highly valuable in industries such as aerospace and automotive manufacturing.
Amphoterism is not limited to inorganic compounds.
While amphoterism is commonly associated with inorganic compounds, certain organic compounds can also exhibit this property. Examples include amino acids, as mentioned earlier, and other biomolecules.
The amphiprotic behavior of substances can be influenced by their molecular structure.
The molecular structure of a substance plays a significant role in determining its amphiprotic behavior. Factors such as the presence of functional groups and the distribution of electron density can influence how a compound interacts with acids and bases.
Amphoterism is a key concept in acid-base chemistry.
Amphoterism is a fundamental concept in acid-base chemistry as it bridges the gap between acidic and basic properties. It helps explain complex reactions and phenomena observed in chemical systems.
Amphoterism can be harnessed in the treatment of acid burns.
Some substances with amphiprotic properties, such as aluminum hydroxide gel, can be used to neutralize acid burns on the skin. The amphoterism of these compounds allows them to react with the excess acid, helping to alleviate the damage caused by the burn.
Certain metal hydrides exhibit amphoterism.
Metal hydrides, such as aluminum hydride (AlH3) and zinc hydride (ZnH2), display amphoterism. They can react with both acids and bases, making them useful in organic synthesis and other chemical reactions.
Amphoteric substances can behave differently in different solvents.
The amphiprotic behavior of substances can vary depending on the solvent they are dissolved in. This phenomenon, known as solvent-dependent amphoterism, highlights the complexity and versatility of this property.
The study of amphoterism is essential in understanding corrosion processes.
Amphoterism plays a significant role in the field of corrosion science. The ability of certain compounds to react with both acids and bases influences the corrosion behavior of metals, leading to the development of protective coatings and corrosion-resistant materials.
Amphoterism is a valuable characteristic in the design of catalytic materials.
The amphiprotic nature of certain materials makes them excellent catalysts for various chemical reactions. By acting as both acids and bases, these catalysts can facilitate the transformation of reactants into desired products.
These 19 intriguing facts about amphoterism shed light on the incredible chemical property that allows certain substances to exhibit dual acid-base behavior. From its applications in various industries to its role in biological processes, amphoterism continues to fascinate scientists and researchers. Understanding and harnessing this property opens up a world of possibilities in the field of chemistry.
So, the next time you come across the term “amphoterism,” remember these fascinating facts and marvel at the unique chemistry behind this extraordinary phenomenon.
Conclusion
In conclusion, amphoterism is a fascinating concept within the field of chemistry. It refers to the ability of certain substances to display both acidic and basic properties, depending on the circumstances. This unique characteristic allows these substances to act as either an acid or a base, depending on the pH of the solution they are placed in.Amphoterism plays a crucial role in various chemical reactions and has significant applications in industries such as pharmaceuticals, metallurgy, and water treatment. Understanding the principles of amphoterism helps chemists design new compounds, predict their behavior, and manipulate their properties to suit specific needs.By delving into the 19 intriguing facts about amphoterism, we have gained a deeper appreciation for the complexity and versatility of these substances. From the discovery of amphoterism to its applications in everyday life, the study of amphoterism continues to expand our knowledge of chemical interactions and opens up new possibilities for scientific advancements.With ongoing research and exploration, we can expect to uncover even more intriguing facts about amphoterism and its implications in the world of chemistry.
FAQs
Q: What are some examples of amphoterism?
A: Some examples of amphoterism include water, aluminum hydroxide, zinc oxide, and amino acids such as glycine.Q: How is amphoterism different from acidity and basicity?
A: Acidity refers to the ability of a substance to donate protons (H+ ions), while basicity refers to the ability to accept protons. Amphoterism, on the other hand, refers to the ability of a substance to exhibit both acidic and basic properties.Q: Can amphoterism be observed in nature?
A: Yes, amphoterism can be observed in natural substances like minerals. For example, minerals like amphoteric oxides and hydroxides display amphoterism when they react with both acids and bases.Q: How does amphoterism impact chemical reactions?
A: Amphoterism allows substances to act as either an acid or a base based on the pH of the solution they are in. This property influences how they interact with other substances and affects the outcome of chemical reactions.Q: Are all substances capable of amphoterism?
A: No, not all substances are amphoterics. Only substances with the appropriate chemical structure and properties can exhibit amphoterism.
Amphoterism's intriguing nature extends beyond these 19 facts. Delving deeper into the unique properties of amphoteric substances reveals even more mindblowing characteristics. From their versatile reactions in different environments to their critical roles in various applications, amphoteric compounds continue to captivate chemists and researchers alike. So, if you're curious to learn more about the fascinating world of amphoterism, keep exploring our collection of articles that shed light on this remarkable phenomenon.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
