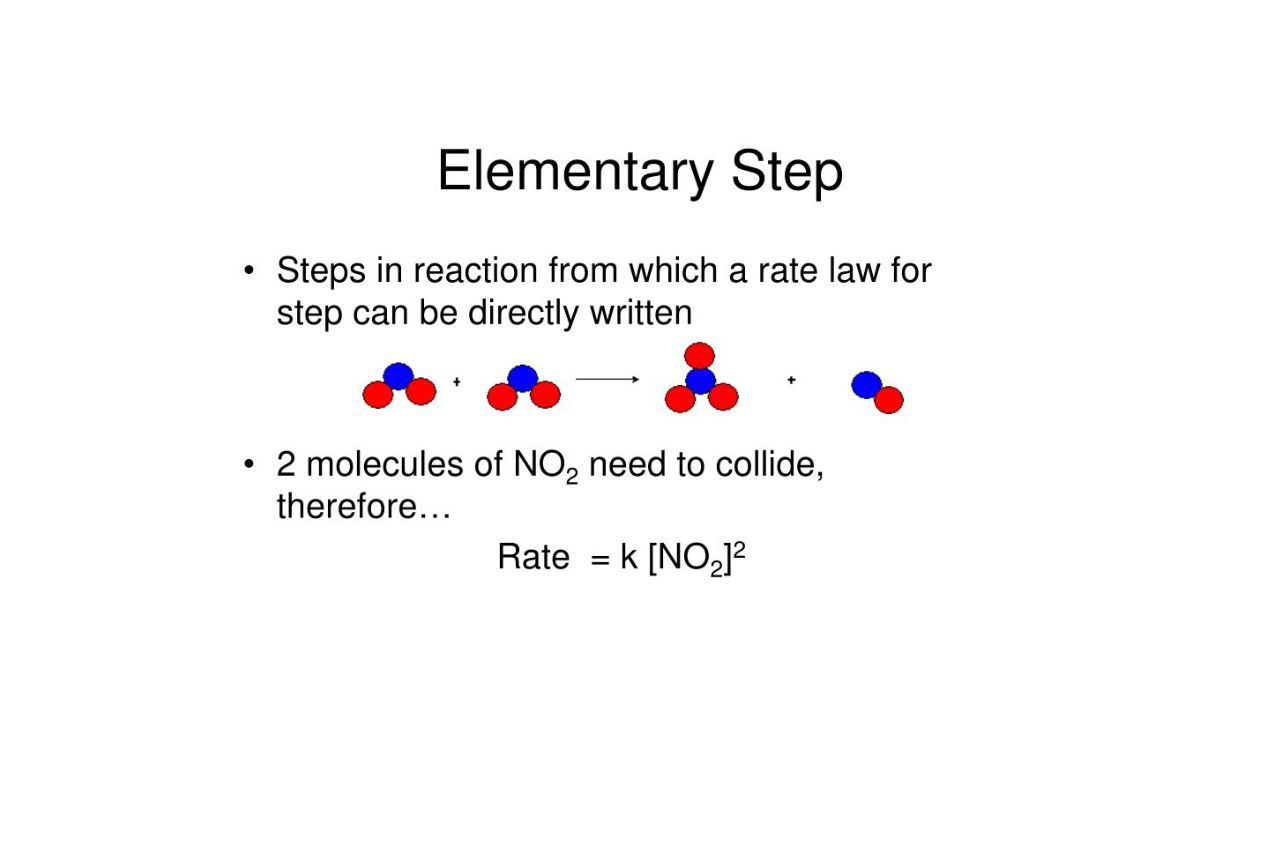
Elementary steps are the fundamental building blocks of chemical reactions, playing a crucial role in the world of chemistry. These steps involve the transfer of atoms, molecules, or electrons between reacting species, paving the way for the formation or breakage of chemical bonds. While elementary steps may seem simple on the surface, they hide a fascinating complexity that unravels the mysteries of chemical reactions.
In this article, we will explore 9 enigmatic facts about elementary steps, delving into their significance in chemical reactions. From understanding the concept of reaction mechanisms to unraveling how catalysts influence the overall reaction, we will dive into the world of elementary steps. So, if you’re ready to embark on a journey through the intricacies of chemical reactions, let’s begin uncovering the secrets of these elementary steps.
Key Takeaways:
- Elementary Step is like a magical dance of molecules, shaping the speed and outcome of chemical reactions. It’s the key to unlocking the secrets of nature and creating new technologies.
- Understanding Elementary Step helps scientists control reactions, design catalysts, and unravel the mysteries of natural processes. It’s the foundation for advancements in medicine, materials, and more.
The Foundational Building Block
Elementary Step serves as the foundational building block of complex chemical reactions. It involves a single reactant transforming into a product through a defined sequence of elementary steps. These steps represent the smallest possible changes in the molecular structure, making them crucial in unraveling the intricacies of chemical transformations.
A Dance of Molecules
Elementary Step can be visualized as a mesmerizing dance of molecules, where reactants collide and form new chemical bonds. This intricate choreography governs the overall rate and outcome of a chemical reaction, making Elementary Step a fascinating subject of study for chemists around the globe.
The Role of Activation Energy
Activation energy acts as the threshold that reactants must overcome to initiate an Elementary Step. This energy barrier determines the speed at which a chemical reaction occurs. By understanding the concept of activation energy, scientists can manipulate and control reactions, opening up avenues for diverse applications in various fields.
Elementary Step and Reaction Mechanisms
Elementary Step plays a crucial role in constructing reaction mechanisms. These mechanisms provide a detailed sequence of Elementary Steps that occur during a chemical reaction. By studying these steps, scientists gain insights into the complex pathways and intermediates involved, further expanding our understanding of chemical processes.
The Fascinating World of Catalysis
Catalysts are substances that accelerate chemical reactions by providing an alternative pathway with lower activation energy. Understanding Elementary Step is essential in designing catalysts that enhance reaction rates and efficiency. This knowledge fuels advancements in industries such as pharmaceuticals, materials science, and environmental sustainability.
The Kinetics of Elementary Step
Elementary Step kinetics focuses on understanding the rates at which individual Elementary Steps occur. By studying the kinetics, scientists can unravel the factors influencing reaction rates, such as temperature, concentration, and catalyst presence. This knowledge is vital for optimizing reaction conditions and developing efficient chemical processes.
Quantum Mechanical Insight
Elementary Step provides quantum mechanical insights into the behavior of atoms and molecules during a chemical reaction. Theoretical calculations and computational models bridge the gap between experimental observations and atomic-level interactions, unraveling the intricate details of Elementary Step and aiding in the design of novel chemical compounds.
The Art of Organic Synthesis
Organic synthesis, the science of creating complex organic compounds, heavily relies on understanding Elementary Step. By strategically designing and manipulating reactions, chemists can construct intricate molecular structures with precision. This expertise fuels advancements in drug discovery, materials science, and the development of new technologies.
Unlocking the Mysteries of Nature
Elementary Step allows us to decipher the intricate mechanisms behind natural phenomena. From the photosynthesis process in plants to the metabolism of living organisms, Elementary Steps serve as the foundation for understanding the fundamental building blocks of life itself.
In conclusion, Elementary Step is more than just a simple concept in chemistry. It unravels the mysteries of chemical reactions and provides the framework for understanding the fundamental processes governing the world around us. By delving into the nine enigmatic facts about Elementary Step, we gain a deeper appreciation for its crucial role in scientific advancements and technological innovations.
Conclusion
In conclusion, the world of chemistry is full of fascinating and enigmatic concepts, and the elementary step is no exception. Understanding the fundamentals of elementary steps is crucial in studying chemical reactions and their mechanisms. The nine facts mentioned above shed light on the intricacies of these steps, their significance, and how they contribute to the overall understanding of chemical processes. Whether it’s grasping the concept of reaction rates, unraveling the mysteries of intermediates, or appreciating the dynamic nature of chemical reactions, the knowledge of elementary steps opens up a whole new world of exploration and discovery in the realm of chemistry.
FAQs
1. What is an elementary step in chemistry?
An elementary step, also known as an elementary reaction, is a chemical reaction that occurs in a single step with a specific rate equation without any intermediate stages. It represents the simplest and most fundamental event in a chemical reaction mechanism.
2. What is the role of elementary steps in chemical reactions?
Elementary steps play a crucial role in understanding the overall reaction mechanism. They help in determining the order of reaction, rate-determining step, and the factors influencing the reaction rate.
3. How are elementary steps related to reaction rates?
The rate of a chemical reaction is determined by the slowest (rate-determining) step in the reaction mechanism. Elementary steps allow us to identify the slowest step, hence providing insights into the overall reaction rate.
4. What are intermediates in elementary steps?
Intermediates are temporary species formed during the course of an elementary step in a chemical reaction. They are neither reactants nor products but are formed and consumed within the reaction mechanism.
5. Can elementary steps occur independently?
Yes, elementary steps can occur independently, but in many cases, they are part of a complex reaction mechanism involving multiple elementary steps.
6. How do elementary steps contribute to the understanding of reaction mechanisms?
By studying elementary steps, researchers can gain valuable information about the sequence of events that occur during a chemical reaction, the intermediates involved, and the overall kinetics of the reaction.
7. Are elementary steps reversible?
Not all elementary steps are reversible. Some can be reversible, while others may only proceed in one direction.
8. Are elementary steps always observable?
No, not all elementary steps are directly observable. Some elementary steps may occur too quickly or involve highly unstable species that are challenging to detect directly.
9. How are elementary steps represented in chemical equations?
Elementary steps are usually represented by individual chemical equations that describe the reactants, products, and stoichiometry involved in the specific step.
Unraveling elementary steps is just the beginning of your journey into chemical reactions. Dive deeper into chemical kinetics and explore the intricacies of reaction mechanisms. Discover how rate-determining steps shape the speed and efficiency of chemical processes. Whether you're a curious learner or a seasoned chemist, there's always more to uncover in the fascinating world of chemistry.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


