Photochemical reactions are fascinating processes that occur when light interacts with matter, triggering a series of chemical transformations. These reactions play a crucial role in various fields such as environmental science, energy production, and even in the synthesis of important compounds in the pharmaceutical industry.
In this article, we will delve into the intriguing world of photochemical reactions and uncover eight astounding facts that will leave you awestruck. From their importance in the Earth’s atmosphere to their impact on our daily lives, photochemical reactions are truly remarkable phenomena that have shaped our world in ways we often overlook.
So, buckle up and get ready to explore the captivating world of photochemical reactions as we dive into these astonishing facts!
Key Takeaways:
- Photochemical reactions are triggered by light and are crucial for natural processes like photosynthesis. Without them, life on Earth wouldn’t be possible!
- Photochemical reactions have diverse applications in everyday life, from sunscreen lotions to glow-in-the-dark materials and solar cells. They’re like the superheroes of chemistry!
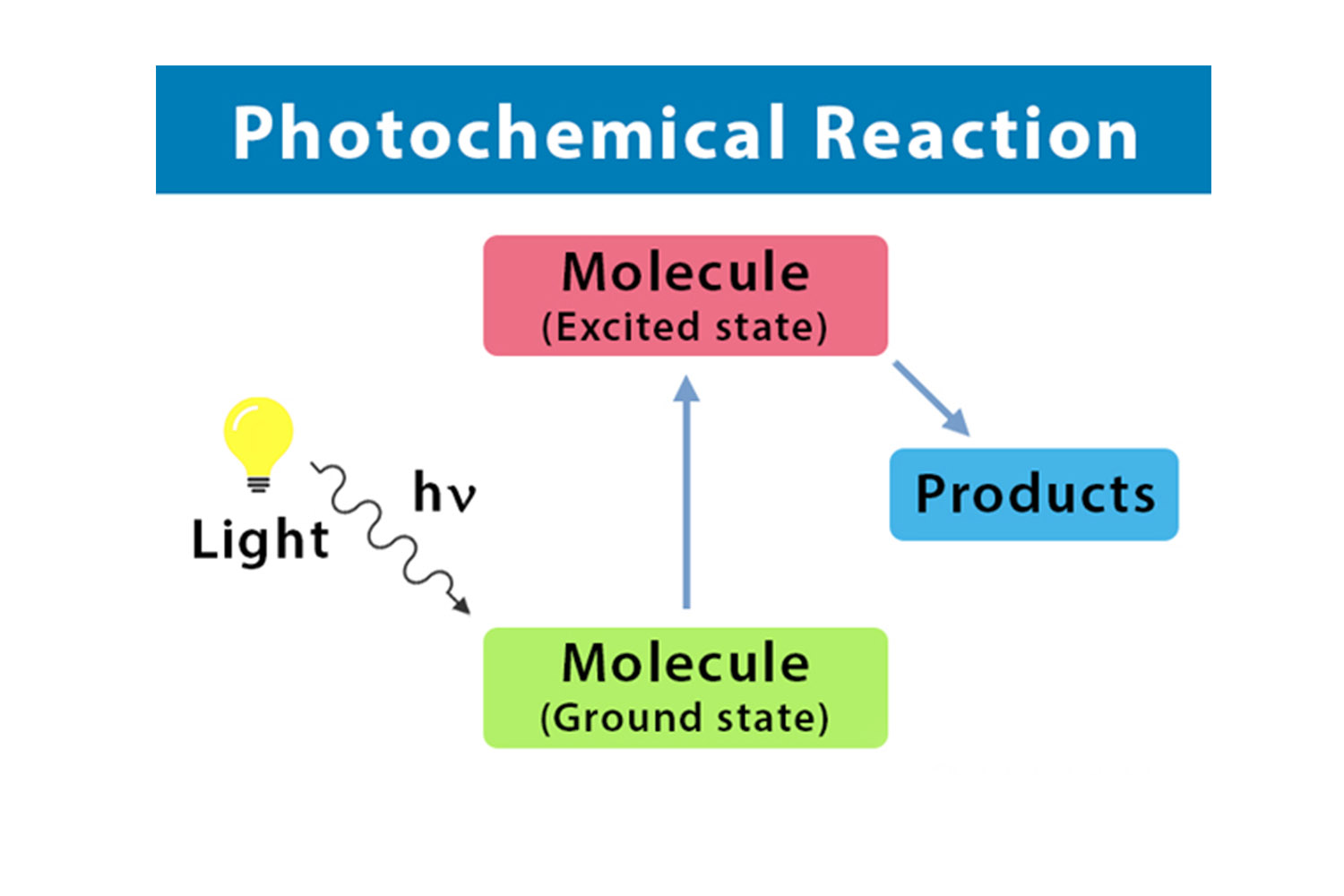
Photochemical reactions are triggered by light.
Photochemical reactions are unique chemical reactions that are initiated by the absorption of light. When suitable molecules absorb photons, they become excited and gain energy, which enables them to undergo chemical transformations.
Photochemical reactions play a crucial role in nature.
Photochemical reactions are essential for various natural processes, such as photosynthesis, the conversion of solar energy into chemical energy in plants. Without photochemical reactions, life on Earth as we know it would not be possible.
Photochemical reactions are responsible for atmospheric ozone depletion.
Certain photochemical reactions involving chlorofluorocarbons (CFCs) have contributed to the depletion of the ozone layer in the Earth’s atmosphere. This depletion has serious consequences for the environment and human health.
Photochemical reactions are used in photography.
In traditional photography, photochemical reactions on light-sensitive film or paper create images. The exposure to light activates the photochemical reactions that result in the formation of a latent image, which can then be developed and fixed to obtain a visible photograph.
Photochemical reactions are employed in chemical synthesis.
Photochemistry has found widespread applications in chemical synthesis, allowing for more efficient and selective reactions. Through the controlled use of light, certain chemical transformations can be achieved that would not be possible through traditional thermal reactions.
Photochemical reactions can cause color changes.
One of the fascinating effects of photochemical reactions is their ability to induce color changes. This phenomenon is often observed in fading of pigments and dyes exposed to light over time.
Photochemical reactions are influenced by various factors.
Several factors can affect the rate and outcome of photochemical reactions, including the intensity and wavelength of light, temperature, pressure, and the presence of catalysts or inhibitors. Understanding these factors is essential for controlling and optimizing photochemical processes.
Photochemical reactions have diverse applications in everyday life.
Photochemical reactions have numerous practical applications, such as sunscreen lotions that protect against harmful UV rays, glow-in-the-dark materials, fluorescent dyes used in biomedical imaging, and solar cells that convert light into electricity.
Conclusion
Photochemical reactions are fascinating phenomena that occur when light interacts with matter, leading to chemical changes. These reactions have numerous applications in various fields, including photography, environmental science, and the development of new materials. By harnessing the power of photochemical reactions, scientists have been able to create efficient solar cells, understand atmospheric processes, and even synthesize complex organic compounds.
Understanding the fundamentals of photochemical reactions is essential for advancing our knowledge of chemistry and unlocking new possibilities for technological advancements. By delving into the astonishing facts surrounding photochemical reactions, we can gain a deeper appreciation for the intricate dance between light and matter.
FAQs
1. What is a photochemical reaction?
A photochemical reaction is a chemical reaction that is initiated by light. It involves the absorption of photons by molecules, which excite the electrons and trigger a series of chemical transformations.
2. How do photochemical reactions differ from thermal reactions?
Photochemical reactions differ from thermal reactions because they are initiated by light energy rather than heat. While thermal reactions rely on the collision of molecules to provide the activation energy, photochemical reactions derive their energy from absorbed photons.
3. What are some examples of photochemical reactions?
Some examples of photochemical reactions include photosynthesis, the formation of ozone in the atmosphere, and the photodegradation of plastics. These reactions play a crucial role in the environment as well as in various industrial processes.
4. How are photochemical reactions used in everyday life?
Photochemical reactions are used in many areas of everyday life. They are employed in photography to create images, in the production of solar panels to convert sunlight into electricity, and in the development of photodynamic therapy for the treatment of cancer.
5. Are all photochemical reactions beneficial?
Not all photochemical reactions are beneficial. Some photochemical reactions, such as the formation of smog, can have detrimental effects on human health and the environment. However, with proper understanding and control, photochemical reactions can be harnessed for positive applications.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


