The Grignard reagent is a class of organometallic compounds that has revolutionized the field of organic chemistry. Named after its discoverer, Victor Grignard, this reagent plays a crucial role in the synthesis of a wide range of organic compounds. It is highly versatile and has been extensively studied and employed by chemists around the world.In this article, we will explore 13 captivating facts about the Grignard reagent. From its discovery to its applications in industry and research, we will delve into the fascinating world of this powerful compound. Whether you are a chemistry enthusiast or a student studying organic chemistry, these facts will deepen your understanding of this important reagent and its impact on the field.Get ready to uncover the secrets of the Grignard reagent and discover why it holds such a significant place in the realm of chemistry.
Key Takeaways:
- Grignard reagent is a powerful compound used in organic chemistry to create complex molecules and natural products. Its versatility and reactivity make it essential for drug synthesis and new chemical discoveries.
- Grignard reagent plays a crucial role in forming carbon-carbon bonds and synthesizing important functional groups like alcohols and amines. It has revolutionized the field of organic chemistry and inspired new synthetic methodologies.
The Grignard reagent is an organometallic compound.
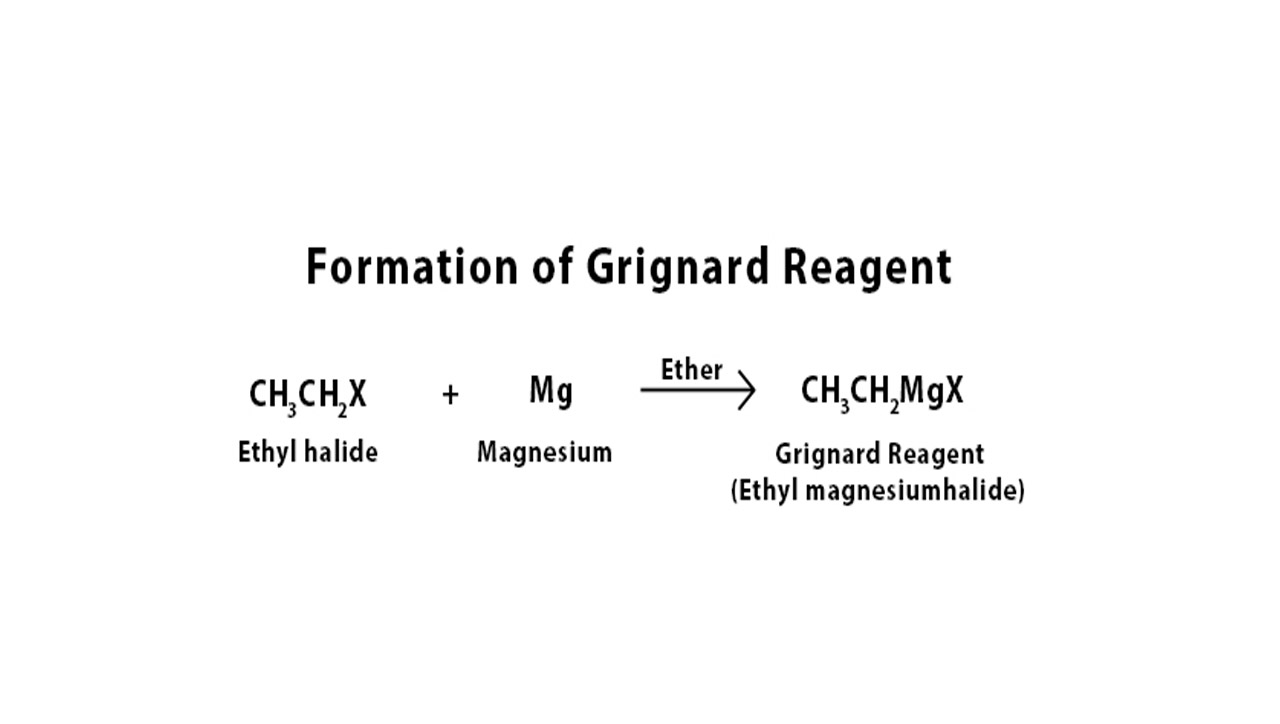
The Grignard reagent is typically an alkyl or aryl magnesium halide compound. It is formed by the reaction of magnesium metal with a halogenated organic compound in anhydrous conditions.
It is highly reactive towards a variety of functional groups.
The Grignard reagent can react with a wide range of functional groups including carbonyl compounds, epoxides, esters, and nitriles. This reactivity makes it a valuable tool for the synthesis of complex organic molecules.
It is used extensively in carbon-carbon bond formation.
The Grignard reagent is commonly employed in reactions that create carbon-carbon bonds. This allows chemists to construct complex carbon frameworks, making it an indispensable tool in organic synthesis.
Grignard reactions are typically conducted in inert atmospheres.
Due to its high reactivity, Grignard reactions are usually carried out under inert atmospheres such as nitrogen or argon to prevent unwanted reactions with moisture or oxygen.
It can be used to prepare alcohols and amines.
Grignard reagents can be reacted with carbonyl compounds to produce alcohols or with nitriles to yield primary amines. This provides chemists with efficient methods for the synthesis of these important functional groups.
It was instrumental in the discovery of new elements.
The Grignard reagent played a crucial role in the discovery of several new elements, including organometallic compounds. This breakthrough has expanded our understanding of the periodic table and opened up new avenues for chemical exploration.
It has applications in pharmaceutical synthesis.
The Grignard reagent is widely utilized in the synthesis of pharmaceutical compounds. Its versatility and ability to form complex carbon frameworks make it an invaluable tool for the development of new drugs and medications.
It can be used in the synthesis of natural products.
The Grignard reagent has been employed in the synthesis of numerous natural products, including alkaloids, terpenes, and steroids. This allows scientists to replicate and study these compounds, unlocking their potential for various applications.
The Grignard reaction follows a nucleophilic addition mechanism.
When the Grignard reagent reacts with a carbonyl compound, it undergoes a nucleophilic addition reaction. The nucleophilicity of the carbon atom bonded to magnesium gives rise to the reactivity of the Grignard reagent.
It can be used to prepare carboxylic acids.
By treating a Grignard reagent with carbon dioxide, carboxylic acids can be synthesized. This transformation, known as the Grignard reaction with carbon dioxide, is a valuable method for the preparation of carboxylic acids.
Grignard reactions are catalyzed by trace amounts of iodine.
In some cases, the addition of iodine as a catalyst enhances the reactivity of the Grignard reagent. This can facilitate the reaction and increase the yield of the desired product.
It is an essential tool in the synthesis of complex natural products.
The ability of the Grignard reagent to form carbon-carbon bonds makes it an indispensable tool in the total synthesis of complex natural products. It enables chemists to construct intricate molecular structures found in nature.
It has paved the way for the development of new synthetic methodologies.
The discovery and development of the Grignard reagent have influenced the field of synthetic chemistry. It has inspired new methodologies, new reactions, and novel approaches to the synthesis of organic compounds.
In conclusion, the 13 captivating facts about the Grignard reagent highlight its significance in organic chemistry. From carbon-carbon bond formations to the synthesis of complex natural products, this versatile reagent has revolutionized the field. Its unique reactivity and wide-ranging applications make it an indispensable tool for chemists worldwide.
Conclusion
In conclusion, Grignard reagents are a fascinating class of compounds with a wide range of applications in organic synthesis. From their serendipitous discovery to their versatility in forming carbon-carbon bonds, these reagents have revolutionized the field of chemistry.Through this article, we have explored 13 captivating facts about Grignard reagents. We learned about their unique structure, their role in nucleophilic addition reactions, and their ability to convert carbonyl compounds into alcohols. We also discovered their significance in pharmaceutical and agrochemical industries, as well as their contribution to the development of new materials.Grignard reagents continue to be indispensable tools for synthetic chemists, enabling the creation of complex molecules with precision and efficiency. As research in organic chemistry advances, it is exciting to anticipate the future discoveries and applications that will further enhance our understanding and utilization of these intriguing compounds.
FAQs
Q: What is a Grignard reagent?
A Grignard reagent is a class of organomagnesium compounds that have the general formula R-Mg-X, where R represents an alkyl or aryl group and X denotes a halogen atom.
Q: How are Grignard reagents prepared?
Grignard reagents are typically prepared by reacting an alkyl or aryl halide with magnesium metal in an anhydrous solvent, such as ether or tetrahydrofuran (THF).
Q: What are the applications of Grignard reagents?
Grignard reagents are widely used in organic synthesis to create new carbon-carbon bonds. They are particularly valuable in the formation of alcohols, as well as in the synthesis of pharmaceuticals, natural products, and fine chemicals.
Q: What are the limitations of Grignard reagents?
Grignard reagents are highly reactive and can react with moisture or protic solvents, leading to undesired side reactions. Additionally, they may not be compatible with certain functional groups, such as acidic protons or electron-withdrawing groups.
Q: Can Grignard reactions be performed on a large scale?
While Grignard reactions are commonly carried out on a laboratory scale, they can also be performed on an industrial scale with careful optimization. Industrial-scale Grignard reactions often involve specialized equipment and safety measures to handle the large quantities of reagents and by-products.
Q: Are there any alternative reagents to Grignard reagents for carbon-carbon bond formation?
Yes, there are several alternative methods for carbon-carbon bond formation, such as transition metal-catalyzed cross-coupling reactions, organolithium reagents, and organozinc reagents. These methods offer complementary approaches to synthesizing complex molecules.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
