The concept of chemical bonding is fundamental to understanding the behavior and properties of molecules. One of the most intriguing types of chemical bonds is the pi bond. Pi bonds play a crucial role in determining the shapes and reactivity of organic molecules.
In this article, we will delve into the captivating world of pi bonds and explore 16 fascinating facts about them. From their discovery to their significance in organic chemistry, we will uncover the unique characteristics and applications of pi bonds.
Whether you’re a chemistry enthusiast, a student, or simply curious about the intricacies of chemical bonding, this article will provide an engaging insight into the captivating world of pi bonds.
Key Takeaways:
- Pi bonds are crucial in chemistry, allowing atoms to share electrons and form double and triple bonds. They play a big role in creating unique shapes and properties in molecules.
- Understanding pi bonds helps scientists create cleaner and more efficient chemical reactions, leading to environmentally friendly processes and sustainable solutions in various industries.
The Basics of Pi Bond
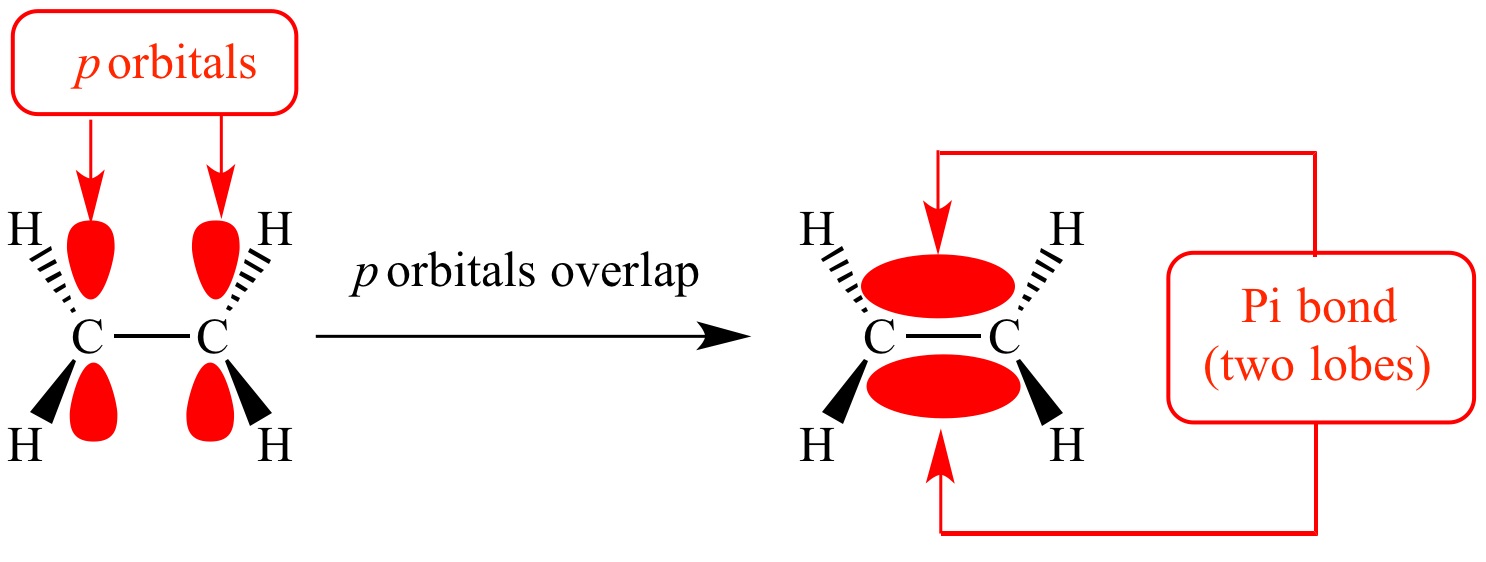
The pi bond is a type of covalent bond in chemistry that occurs when two atomic orbitals overlap to share electrons. It is denoted by the Greek letter ? and is a crucial component in the formation of double and triple bonds.
The History of the Pi Bond
The concept of the pi bond was introduced by Sir Robert Robinson in He proposed the idea to explain the structure and behavior of certain organic compounds.
The Electrons Involved
In a pi bond, overlapping of p orbitals occurs, and electrons are shared between the atoms involved. This sharing of electrons allows for the formation of multiple bonds and is vital for the stability of many organic molecules.
Types of Pi Bonds
There are two types of pi bonds: the pi bond formed by the side-by-side overlap of p orbitals, known as a pi bond, and the pi bond formed by the overlap of a hybridized orbital and a p orbital, known as a pi-lone pair bond.
Strength of Pi Bond
The strength of a pi bond depends on various factors, including bond length and the extent of overlap between the atomic orbitals. Generally, pi bonds are weaker than sigma bonds but are crucial for the stability and reactivity of many molecules.
Role in Molecular Geometry
The presence of pi bonds affects the molecular geometry of a compound. Double and triple bonds result in a different spatial arrangement compared to single bonds, leading to unique shapes and properties.
Pi Bond and Aromaticity
Pi bonds play a significant role in the formation of aromatic compounds. Aromaticity refers to a special type of stability exhibited by some planar, cyclic compounds that contain conjugated pi systems.
Delocalization of Pi Electrons
One of the remarkable properties of pi bonds is the ability of the electrons to delocalize. This means that the electrons are not localized between two specific atoms but are spread out over a larger region of the molecule.
Pi Bond in Alkenes
Alkenes are hydrocarbon molecules that contain a carbon-carbon double bond. This double bond consists of one sigma bond and one pi bond, and it gives alkenes unique chemical reactivity.
Pi Bond in Alkynes
Alkynes are hydrocarbon molecules that contain a carbon-carbon triple bond. This triple bond consists of one sigma bond and two pi bonds, making alkynes even more reactive than alkenes.
Pi Bond and Resonance
The presence of pi bonds allows for resonance stabilization in certain compounds. Resonance refers to the delocalization of electrons across different atoms or bonds in a molecule, resulting in increased stability.
Applications in Organic Synthesis
The pi bond plays a crucial role in organic synthesis, as it enables the formation of complex molecules. Chemists utilize the reactivity and versatility of pi bonds to create a wide range of pharmaceuticals, polymers, and other useful compounds.
The Pi Bond in Benzene
Benzene is a prime example of a compound that contains multiple pi bonds. Its molecular structure consists of a ring of six carbon atoms, each contributing one pi bond to create an aromatic system.
Pi Bond and Molecular Orbitals
In molecular orbital theory, the pi bond is formed by the overlap of atomic orbitals to create bonding and antibonding molecular orbitals. These molecular orbitals determine the electronic structure and behavior of the molecule.
Research and Advancements in Pi Bond Chemistry
The study of pi bonds and their role in various chemical processes is an area of active research. Scientists are continually expanding their understanding of the pi bond and finding new applications and advancements in this field.
Social and Environmental Impact
The understanding of pi bonds and their role in organic chemistry is crucial for developing environmentally friendly processes and sustainable solutions in various industries. By utilizing the unique reactivity of pi bonds, researchers can design cleaner and more efficient chemical reactions.
Conclusion
In conclusion, Pi bonds are a fascinating aspect of chemistry that play a crucial role in molecular structure and bonding. They contribute to the stability and reactivity of organic compounds and have significant implications in various fields, such as pharmaceuticals, materials science, and biochemistry. Understanding the concept of Pi bonds provides valuable insights into the nature of chemical reactions and the properties of different compounds.Pi bonds are formed by the overlap of p orbitals, which leads to the formation of double or triple bonds in molecules. They exhibit unique characteristics, such as delocalization and the ability to participate in resonance structures, which contribute to their stability and versatility.Exploring the world of Pi bonds opens up a whole new dimension in chemistry, allowing us to delve deeper into the intricacies of molecular structures and interactions. As our understanding of Pi bonds continues to evolve, it will undoubtedly lead to more advancements and discoveries in the field of chemistry.
FAQs
Q: What is a Pi bond?
A: A Pi bond is a type of covalent bond formed by the overlap of p orbitals in adjacent atoms.
Q: How does a Pi bond differ from a sigma bond?
A: A sigma bond is a direct overlap of atomic orbitals, whereas a Pi bond is a sideways overlap of p orbitals.
Q: What is the significance of Pi bonds in organic chemistry?
A: Pi bonds play a crucial role in the stability, reactivity, and electronic properties of organic compounds.
Q: Can Pi bonds participate in resonance structures?
A: Yes, Pi bonds can participate in resonance structures, which contribute to the stability and delocalization of electrons.
Q: Are Pi bonds found only in organic compounds?
A: While Pi bonds are commonly found in organic compounds, they can also exist in inorganic molecules and coordination complexes.
Q: How do Pi bonds contribute to the properties of materials?
A: Pi bonds influence the electronic and bonding properties of materials, affecting factors such as conductivity, optical properties, and mechanical strength.
Exploring the captivating world of pi bonds is just the beginning! Dive deeper into the fascinating realm of chemistry and uncover a wealth of knowledge. Curious about the intricacies of molecular bonding? Our article on pi molecular orbitals will satisfy your curiosity. If you're intrigued by the unique properties of double bonds, don't miss our piece on alkenes. Embark on a journey of discovery and expand your understanding of the chemical world around us.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


