Valence electron configuration is a fundamental concept in chemistry that plays a crucial role in understanding the behavior and properties of elements. These electrons, found in the outermost energy level of an atom, determine its reactivity and the way it interacts with other atoms. Understanding the valence electron configuration helps chemists predict the formation of chemical bonds, the stability of compounds, and even the colors and magnetic properties of substances.
In this article, we will delve into the intriguing world of valence electron configuration and explore 20 fascinating facts about this concept. From the significance of electron shells to the octet rule, electronegativity, and hybridization, we will uncover the secrets behind chemical bonding and its impact on the properties of various elements and compounds. Whether you are a student, a chemistry enthusiast, or simply curious about the wonders of the atomic world, prepare to be amazed by these engaging facts about valence electron configuration!
Key Takeaways:
- Valence electron configuration determines how elements bond and react, influencing their chemical properties and behavior in the periodic table.
- The number and arrangement of valence electrons impact an element’s ability to form different types of chemical bonds and participate in various reactions.
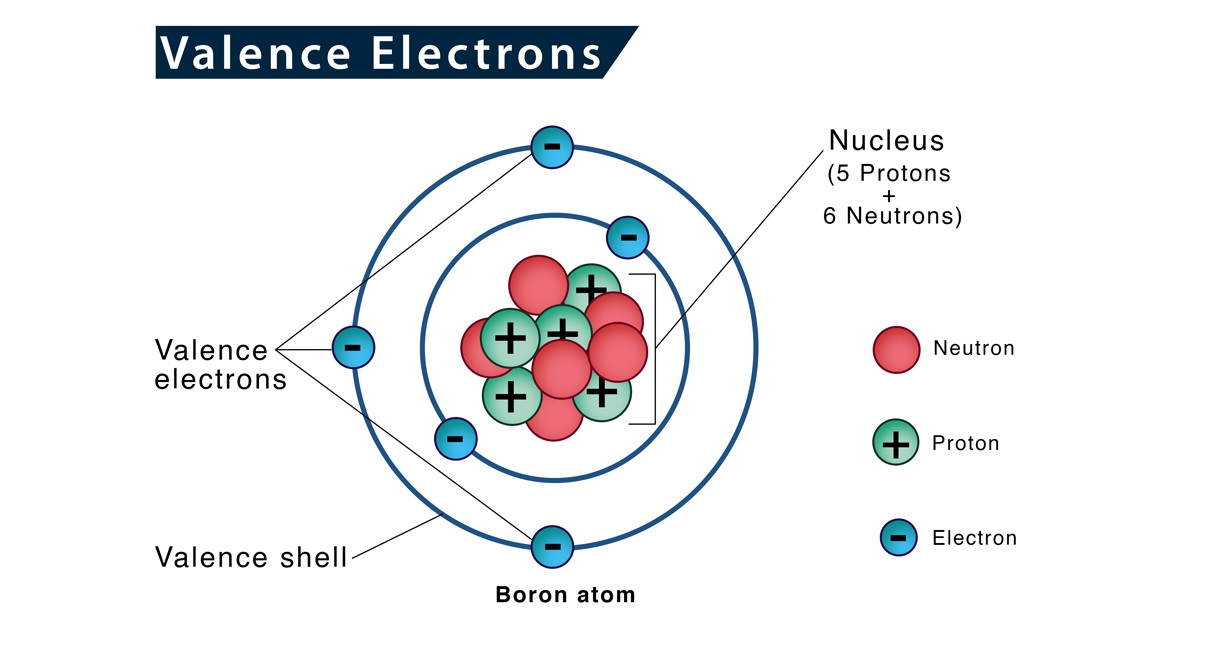
What is Valence Electron Configuration?
Valence electron configuration refers to the distribution of electrons in the outermost energy level, also known as the valence shell, of an atom. It determines the chemical properties and reactivity of an element.
Valence Electrons are Important
Valence electrons play a crucial role in the bonding of atoms and the formation of chemical compounds. Their interaction determines how atoms combine to form molecules.
Valence Electrons Determine the Periodic Table
The number of valence electrons determines an element’s position in the periodic table and its chemical behavior. Elements with the same number of valence electrons are grouped together.
Valence Electrons Follow the Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight electrons in the valence shell, resembling the electron configuration of noble gases.
Transition Metals Have Variable Valence Electrons
Unlike main group elements, transition metals have variable valence electrons, meaning they can lose different numbers of electrons and form multiple positive charges.
Valence Electrons Determine Chemical Reactivity
The number and arrangement of valence electrons determine an element’s ability to participate in chemical reactions and bond with other elements.
Valence Electrons Influence Bonding Types
Based on their valence electron configuration, elements can form ionic, covalent, or metallic bonds, resulting in different types of chemical compounds.
Noble Gases Have Stable Valence Electron Configurations
Noble gases have full valence shells and are chemically unreactive due to their stable electron configurations. They rarely form compounds with other elements.
Valence Electrons Determine Oxidation States
Valence electrons are involved in the determination of an element’s oxidation states or charges in chemical compounds.
Lewis Dot Diagrams Illustrate Valence Electron Configuration
Lewis dot diagrams, also known as electron dot diagrams, use dots to represent valence electrons around atomic symbols, providing a visual representation of an element’s valence electron configuration.
Valence Electrons Impact Bond Length and Strength
The number of valence electrons affects the bond length and strength of a chemical bond. More valence electrons may result in shorter and stronger bonds.
Valence Electrons Determine Molecular Shapes
The arrangement of valence electrons influences the molecular shape and geometry of compounds, which in turn affects their chemical properties and interactions.
Valence Electrons are Involved in Electron Transfer
During redox reactions, valence electrons are transferred between atoms, resulting in the formation of ions with different electron configurations.
Valence Electrons Contribute to the Color of Elements
The interaction of valence electrons with light causes elements to exhibit different colors. This property is used in spectroscopy to identify elements and compounds.
Electronegativity is Influenced by Valence Electrons
Valence electrons contribute to an element’s electronegativity, which measures its ability to attract electrons in a chemical bond.
Valence Electrons Determine Chemical Stability
An element’s valence electron configuration influences its chemical stability. Elements with full valence shells tend to be more stable and less reactive.
Valence Electrons Control Conductivity
In metallic elements, valence electrons are free to move, contributing to high electrical and thermal conductivity.
Energy Levels Determine the Number of Valence Electrons
The number of valence electrons is determined by the energy level or period an element belongs to in the periodic table.
Valence Electrons Play a Role in Semiconductor Behavior
In semiconductors, the manipulation of valence electrons allows for the control of conductivity, which is essential for modern electronics.
Valence Electron Configuration has Implications in Chemical Bonding
The unique valence electron configuration of an element influences the types of chemical bonds it can form, such as ionic, covalent, or metallic bonds.
Conclusion
In conclusion, understanding valence electron configuration is crucial in comprehending the behavior and properties of elements. The arrangement of valence electrons determines an element’s reactivity, chemical bonding, and overall stability. By studying the valence electron configuration, scientists can predict an element’s behavior and its ability to form compounds.
Valence electron configuration is a fascinating aspect of chemistry that provides valuable insights into the periodic table and its trends. From the unique electron configurations of noble gases to the varying electron arrangements of transition metals, valence electron configuration allows us to understand the diverse properties and characteristics of elements.
Exploring the intriguing world of valence electron configuration not only deepens our appreciation for the complexity of chemistry but also enhances our understanding of the fundamental building blocks of matter.
FAQs
Q: What are valence electrons?
A: Valence electrons are the electrons in the outermost energy level of an atom. They are responsible for an element’s chemical reactivity and bonding behavior.
Q: How do you determine the valence electron configuration of an element?
A: The valence electron configuration can be determined by examining an element’s position in the periodic table and identifying the number of electrons in its outermost energy level.
Q: Why is valence electron configuration important?
A: Valence electron configuration is important because it determines how atoms interact with each other, leading to the formation of compounds and the behavior of elements in chemical reactions.
Q: What is the significance of the octet rule in valence electron configuration?
A: The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons, similar to the noble gases.
Q: How does valence electron configuration affect an element’s chemical properties?
A: The number and arrangement of valence electrons influence an element’s ability to bond with other atoms, its reactivity, and its overall chemical behavior.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
