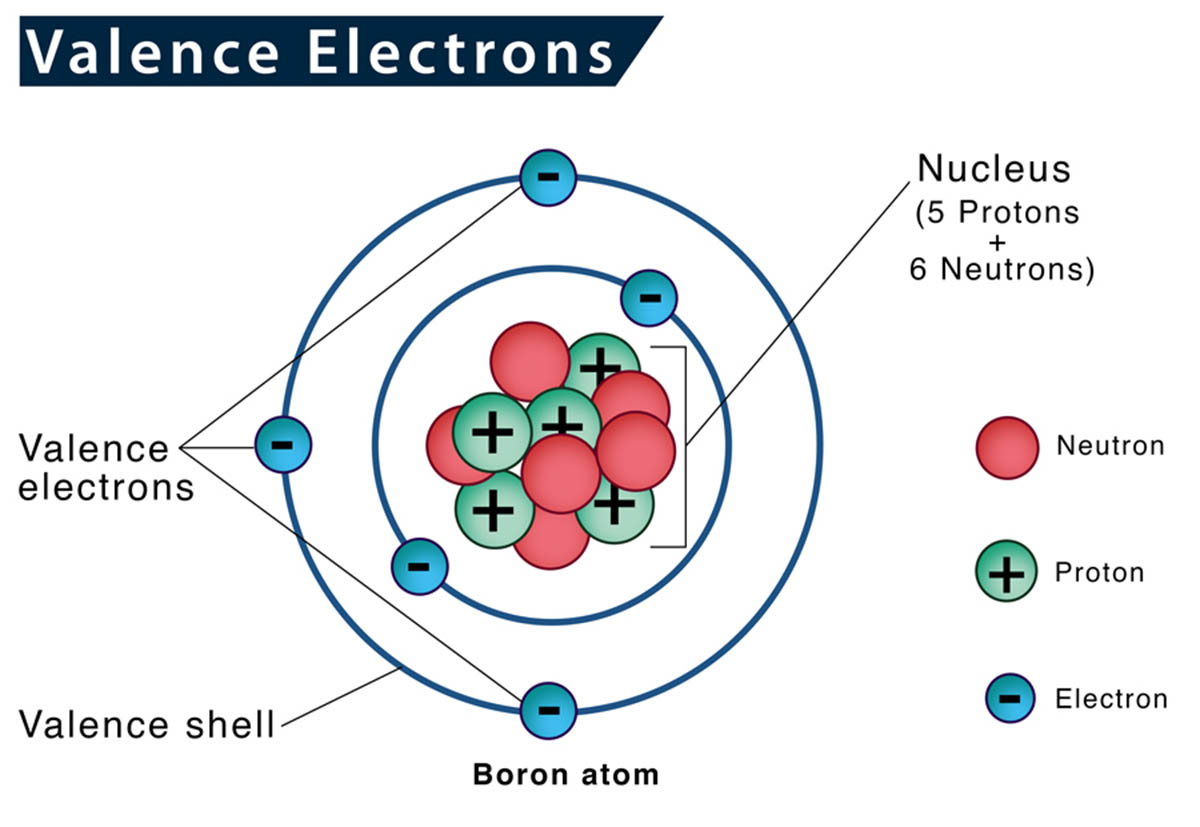
Valence electrons are an essential component of chemistry and play a crucial role in understanding the behavior and properties of atoms and molecules. These electrons are located in the outermost energy level of an atom and are responsible for bonding and chemical reactions. Exploring the world of valence electrons opens up a fascinating realm of knowledge, revealing the intricacies of chemical bonding, reactivity, and the formation of compounds.
In this article, we will dive into the intriguing world of valence electrons and uncover 14 fascinating facts that will enhance your understanding of these enigmatic particles. From their relationship to the periodic table to their impact on chemical reactions, we will explore the significance of valence electrons in the study of chemistry. So, grab your lab coat and safety goggles as we embark on this electrifying journey into the world of valence electrons!
Key Takeaways:
- Valence electrons determine how elements behave and bond with each other. They are like the “social butterflies” of the atom, influencing reactivity, bonding, and even the color of compounds.
- The number of valence electrons an element has affects its chemical reactivity and physical properties. It’s like the “personality” of the element, determining how it interacts with others and what it looks like.
The Definition of Valence Electron
A valence electron is an electron that is located in the outermost energy level, or shell, of an atom. These electrons are involved in chemical bonding and determine the chemical properties of an element.
Valence Electrons Determine the Reactivity of an Element
The number of valence electrons in an atom determines its reactivity. Elements with one or two valence electrons tend to be highly reactive, while those with a full valence shell are stable and unreactive.
Valence Electrons Play a Key Role in Bonding
Valence electrons are responsible for the formation of chemical bonds between atoms. They can be shared, gained, or lost in order to achieve a stable electron configuration.
Valence Electrons Determine the Element’s Position on the Periodic Table
The number of valence electrons corresponds to an element’s position in the periodic table. Elements in the same group have similar valence electron configurations and exhibit similar chemical behavior.
Valence Electrons Influence the Physical Properties of Elements
The presence or absence of valence electrons affects an element’s melting point, boiling point, conductivity, and other physical properties. For example, metals tend to have few valence electrons, making them good conductors of electricity.
Valence Electrons Contribute to the Formation of Ions
When atoms gain or lose valence electrons, they become ions. Positive ions are formed when atoms lose valence electrons, while negative ions are formed when atoms gain valence electrons.
Valence Electrons Determine the Oxidation State of an Atom
The oxidation state of an atom in a compound is determined by the number of valence electrons it gains, loses, or shares. This is important in understanding chemical reactions and balancing chemical equations.
The Octet Rule Relates to Valence Electrons
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight valence electrons. This rule explains why many elements form compounds with a noble gas electron configuration.
Transition Metals Have Variable Valence Electrons
Unlike main group elements, transition metals can have multiple valence electrons. This allows them to exhibit a wide range of oxidation states and participate in various types of chemical bonding.
Valence Electron Configurations Can Predict Chemical Reactivity
By knowing the number of valence electrons in an element, we can predict its chemical reactivity. Elements with fewer valence electrons are more likely to gain or share electrons, while those with more valence electrons are more likely to lose them.
Valence Electrons Determine the Lewis Structure of a Molecule
The Lewis structure of a molecule shows the arrangement of atoms and their valence electrons. It helps us understand the bonding and shape of molecules, which is crucial in studying chemical reactions.
Valence Electrons Influence the Color of Transition Metal Complexes
The presence of d-block transition metals and their partially filled d-orbitals in compounds contribute to their vibrant colors. The absorption and reflection of light by these valence electrons lead to the observed colors.
The Study of Valence Electrons Falls Under the Field of Chemical Bonding
Chemical bonding is a branch of chemistry that focuses on the interactions between atoms and the formation of compounds. The study of valence electrons plays a central role in understanding chemical bonding.
The Valence Electrons of Noble Gases Are Complete
Noble gases have a full outer shell of valence electrons, making them stable and unreactive. This is why noble gases are often used in applications that require inert atmospheres, such as lighting and welding.
Conclusion
In conclusion, valence electrons are a fascinating aspect of chemistry. They play a crucial role in determining the chemical behavior and properties of elements. Understanding valence electrons allows us to predict bonding patterns, the formation of compounds, and the reactivity of elements.Valence electrons are the outermost electrons in an atom’s electron cloud. They participate in chemical reactions by either gaining, losing, or sharing electrons to achieve a stable electron configuration. The number of valence electrons determines an element’s group on the periodic table and influences its chemical behavior.By studying valence electrons, scientists can design materials with specific properties, create new compounds, and develop innovative technologies. Valence electron theory has applications in various fields, including medicine, energy, and materials science.Continued research on valence electrons will undoubtedly lead to further advances in chemistry and our understanding of the natural world. It is a captivating area of study that continues to unveil new insights into the building blocks of matter.
FAQs
Q: What is a valence electron?
A: Valence electrons are the outermost electrons in an atom’s electron cloud. They are involved in chemical reactions and interact with other atoms to form compounds.
Q: How do valence electrons determine chemical behavior?
A: The number of valence electrons influences the bonding patterns and reactivity of elements. It determines an element’s group on the periodic table and provides insight into its chemical properties.
Q: How do atoms achieve a stable electron configuration?
A: Atoms achieve a stable electron configuration by gaining, losing, or sharing electrons. This process allows them to fill their outermost energy level and achieve a more stable state.
Q: What is the significance of studying valence electrons?
A: Studying valence electrons allows scientists to understand and predict the behavior of elements, design new compounds, and develop innovative technologies in various fields, such as medicine, energy, and materials science.
Q: How does valence electron theory contribute to scientific advancements?
A: Valence electron theory provides a foundation for understanding chemical reactions, bonding, and the properties of compounds. It forms the basis for advancements in fields like materials science and helps drive scientific discoveries and technological breakthroughs.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
