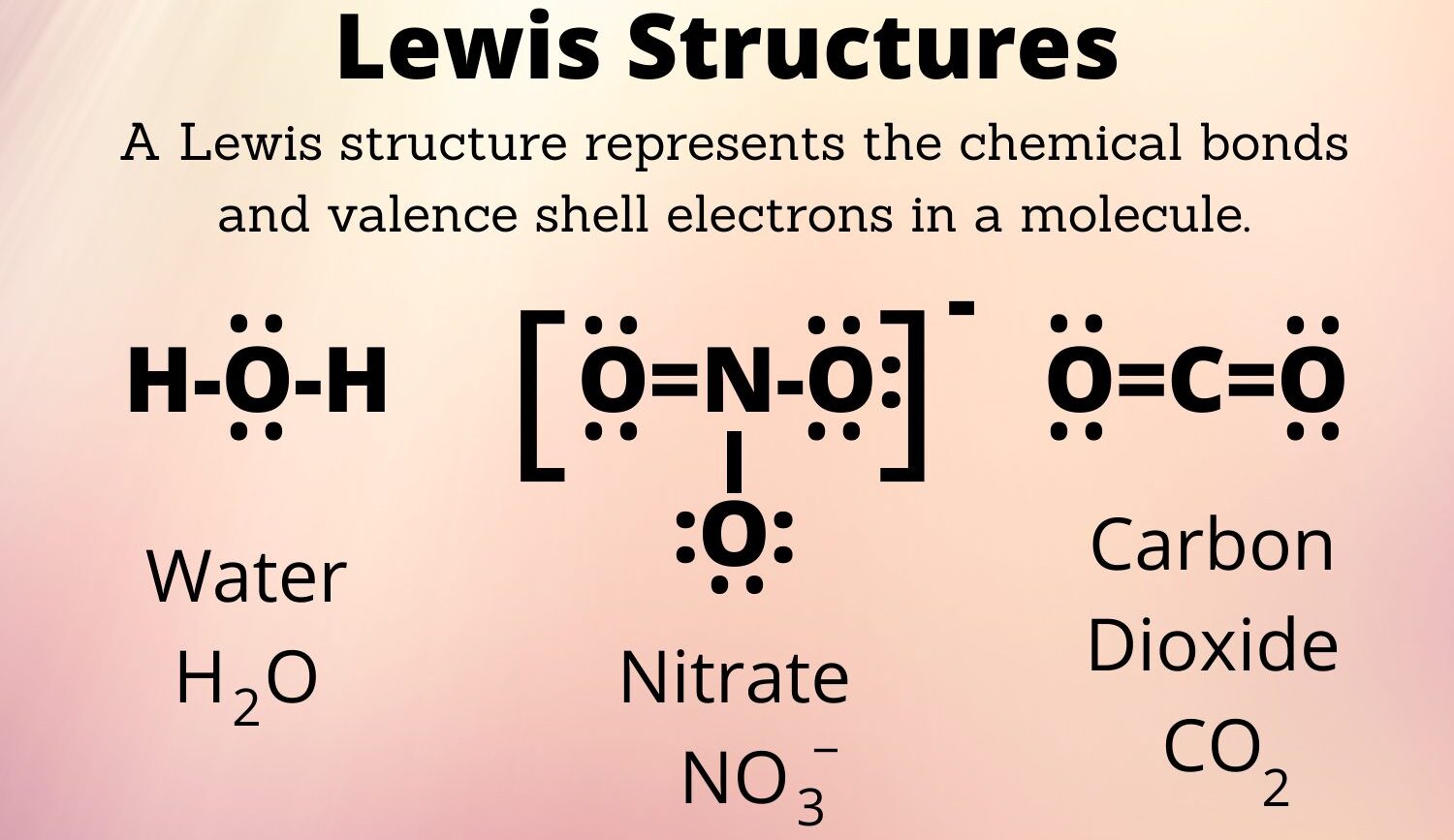
The Lewis structure is a fundamental concept in chemistry that helps us understand the bonding and structure of molecules. Developed by American chemist Gilbert N. Lewis in the early 20th century, the Lewis structure provides a visual representation of how atoms are connected in a molecule and the arrangement of electrons within it. Understanding Lewis structures is crucial in various areas of chemistry, including organic chemistry, inorganic chemistry, and biochemistry.
In this article, we will explore eleven fascinating and mind-boggling facts about Lewis structures. From their significance in determining molecular geometry to their role in chemical reactions, these facts will deepen your understanding of this essential concept. So, let’s dive in and discover the unbelievable world of Lewis structures!
Key Takeaways:
- Lewis structures visually represent how atoms bond in molecules, helping us understand their shape, properties, and reactivity. They show us the secrets of chemical compounds in a cool and visual way!
- By using Lewis structures, we can predict how atoms in molecules will behave, understand their electron distribution, and even use computer programs to make complex diagrams. It’s like a secret code to unlock the mysteries of chemistry!
The 11 Unbelievable Facts About Lewis Structure
Lewis structure is a fundamental concept in chemistry that helps us understand the arrangement of atoms and the bonding in a molecule. From its discovery by Gilbert N. Lewis in 1916, it has revolutionized the way we study and comprehend chemical compounds. Here are 11 mind-blowing facts about the Lewis structure that will leave you amazed!
The Lewis structure provides a visual representation of a molecule’s bonding
The Lewis structure is a diagrammatic representation of a molecule that illustrates the valence electrons and their arrangement. By using dots to represent electrons and lines to represent bonds, it showcases the sharing or transfer of electrons between atoms, giving us a clear picture of how atoms are connected within a molecule.
It allows us to predict the geometry and properties of a molecule
By analyzing the Lewis structure, we can determine the molecular geometry, which in turn helps us understand the physical and chemical properties of the compound. The arrangement of atoms and lone pairs of electrons around the central atom dictates the shape, polarity, and reactivity of the molecule.
The octet rule is a guiding principle in Lewis structure
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eight electrons in their valence shell. Lewis structures help us visualize how atoms achieve an octet by either forming single, double, or triple bonds or by gaining or losing electrons.
Lewis structure aids in the understanding of chemical reactions
By depicting the electron distribution and bond formation, Lewis structures provide insights into how chemical reactions occur. They help us analyze the breaking and forming of bonds, electron rearrangements, and the overall transformation of compounds during a reaction.
Resonance structures provide alternative representations of a molecule
In cases where multiple Lewis structures can be drawn for a molecule, resonance structures come into play. Resonance occurs when electron delocalization is possible, resulting in several valid arrangements. These resonance structures contribute to the overall stability and reactivity of the molecule.
The Lewis structure can be used to predict the hybridization of atoms
By examining the Lewis structure, we can determine the hybridization of atoms in a molecule. Hybridization refers to the mixing of atomic orbitals to form new hybrid orbitals, which influences the geometry and bonding characteristics of the molecule.
Lewis structures help in understanding the concept of formal charge
Formal charge is a concept used to evaluate the distribution of electrons in a molecule. By comparing the number of valence electrons an atom possesses in its neutral state to the number it has in the Lewis structure, we can determine the formal charge. This helps us understand the electron distribution and stability within the molecule.
Electronegativity plays a crucial role in Lewis structure
Electronegativity is the measure of an atom’s ability to attract electrons towards itself in a chemical bond. It is a vital concept in Lewis structure as it helps us determine the polarity of bonds and the distribution of electrons between atoms.
The Lewis structure can be expanded to include multiple elements
While Lewis structures are commonly associated with organic molecules, they can also be applied to compounds containing multiple elements. This provides a way to represent complex structures involving various types of atoms, allowing for a better understanding of their bonding and electron distribution.
Computer software assists in generating complex Lewis structures
As molecules become more intricate, manually constructing Lewis structures can be challenging. Luckily, there are computer programs available that utilize algorithms to generate complex structure diagrams quickly and accurately. These software tools aid chemists in studying and analyzing increasingly complex chemical systems.
These 11 unbelievable facts about Lewis structure highlight the significance and versatility of this fundamental concept in chemistry. They showcase how Lewis structures provide us with valuable insights into molecular bonding, geometry, and reactivity, enabling us to unravel the mysteries of the chemical world.
Conclusion
In conclusion, Lewis structures are a fascinating topic in chemistry. They provide a visual representation of how atoms are connected in a molecule and play a crucial role in understanding the behavior and properties of compounds.
Throughout this article, we’ve explored several unbelievable facts about Lewis structures. From their invention by Gilbert N. Lewis to their importance in predicting molecular geometry, these structures continue to revolutionize the study of chemistry.
Understanding Lewis structures can be challenging at first, but with practice and knowledge of valence electrons, you’ll be able to create accurate representations of molecules in no time. So, dive into the world of Lewis structures and unlock the secrets of chemical bonding!
FAQs
Q: What is a Lewis structure?
A: A Lewis structure, also known as a Lewis dot structure or electron dot structure, is a visual representation of the arrangement of atoms and valence electrons in a molecule.
Q: How are Lewis structures useful?
A: Lewis structures help chemists understand the connectivity and bonding in molecules, including predicting molecular geometry, determining bond polarity, and identifying formal charges.
Q: Can Lewis structures accurately depict the 3D shape of a molecule?
A: Lewis structures provide a simplified 2D representation of molecules and do not always accurately depict the 3D shape. However, they serve as a starting point for understanding molecular geometry.
Q: How are Lewis structures drawn?
A: Lewis structures are drawn by representing atoms as symbols and using dots or lines to represent valence electrons needed to complete the octet rule. Lone pairs of electrons are typically represented as dots.
Q: Are there any exceptions to the octet rule in Lewis structures?
A: Yes, there are certain elements, such as hydrogen (H), boron (B), and elements beyond the third period in the periodic table, that can have fewer than eight valence electrons and still be stable.
Q: Can Lewis structures be used for all molecules?
A: Lewis structures are primarily used for covalent compounds, where electrons are shared between atoms. They are not commonly used for ionic compounds, which involve the transfer of electrons.
Q: How can I determine the Lewis structure of a molecule?
A: To determine the Lewis structure of a molecule, first, count the total number of valence electrons. Then, determine how the atoms are connected and distribute the valence electrons to satisfy the octet rule.
Q: Can I have multiple Lewis structures for a molecule?
A: Yes, some molecules can have multiple valid Lewis structures. This phenomenon is known as resonance and occurs when two or more Lewis structures can be drawn for a molecule without changing its overall charge or arrangement of atoms.
Q: Are there any exceptions to the octet rule?
A: Yes, there are molecules with an odd number of electrons or an expanded octet where more than eight electrons can surround an atom, such as in phosphorus pentachloride (PCl5) or sulfur hexafluoride (SF6).
Q: Can I determine the polarity of a molecule from its Lewis structure?
A: By considering the electronegativity difference between atoms and the molecular geometry, you can infer the polarity of a molecule from its Lewis structure. However, it is essential to verify the molecule’s polarity experimentally.
Q: How can I practice drawing Lewis structures?
A: Practice drawing Lewis structures by working on exercises and examples. Familiarize yourself with the periodic table, electron configurations, and the octet rule. Online resources, textbooks, and chemistry tutorials can also provide additional guidance.
Unraveling Lewis structures' secrets is just the beginning! Delving deeper into chemical bonding, you'll find even more captivating facts about these fundamental diagrams. Formal charges play a crucial role in determining molecular stability, while resonance structures offer alternative representations of electron distribution. Exploring these concepts further will enhance your understanding of chemistry and its myriad applications. So, buckle up and prepare for an exciting journey through the world of chemical bonding!
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
