The field of chemistry is full of fascinating concepts and theories that help us understand the intricacies of matter and its behavior. One such theory that has revolutionized our understanding of chemical reactions is the collision theory. In simple terms, the collision theory states that chemical reactions occur when reactant particles collide with sufficient energy and proper orientation. However, there are many astonishing facts about collision theory that make it even more intriguing. From its historical development to its practical applications in various industries, collision theory continues to be a cornerstone in the study of chemical kinetics. In this article, we will explore 19 astonishing facts about collision theory, revealing its significance and impact on the world of chemistry. So, buckle up as we dive into this fascinating theory and uncover its hidden gems.
Key Takeaways:
- Collision theory explains how chemical reactions happen when molecules collide with enough energy and the right orientation. It helps scientists understand and predict reaction rates, influencing everything from industrial processes to biological reactions.
- By studying collision theory, scientists can optimize reaction conditions, validate kinetic models, and gain insights into reaction mechanisms. It’s like uncovering the secret code of chemical reactions, opening doors to new discoveries in chemistry and beyond.
The Origin of Collision Theory
The concept of collision theory was first proposed by Max Trautz and William Lewis in They suggested that chemical reactions occur when reactant molecules collide with sufficient energy and in the correct orientation. This theory laid the foundation for our understanding of chemical kinetics.
The Role of Activation Energy
According to collision theory, for a chemical reaction to occur, the colliding molecules must possess a minimum amount of energy called activation energy. This energy is required to break the existing bonds in the reactant molecules and form new bonds in the product molecules.
Factors Affecting Reaction Rate
Collision theory explains that several factors influence the rate of a chemical reaction. These include concentration, temperature, surface area, and the presence of catalysts. Increasing the concentration of reactant molecules, raising the temperature, and using a catalyst can all increase the frequency of collisions and thus accelerate the reaction rate.
The Effect of Temperature
One of the key principles of collision theory is that increasing the temperature generally leads to an increase in the reaction rate. This is because higher temperatures result in higher kinetic energy of the molecules, increasing the likelihood of successful collisions.
Orientational Requirements
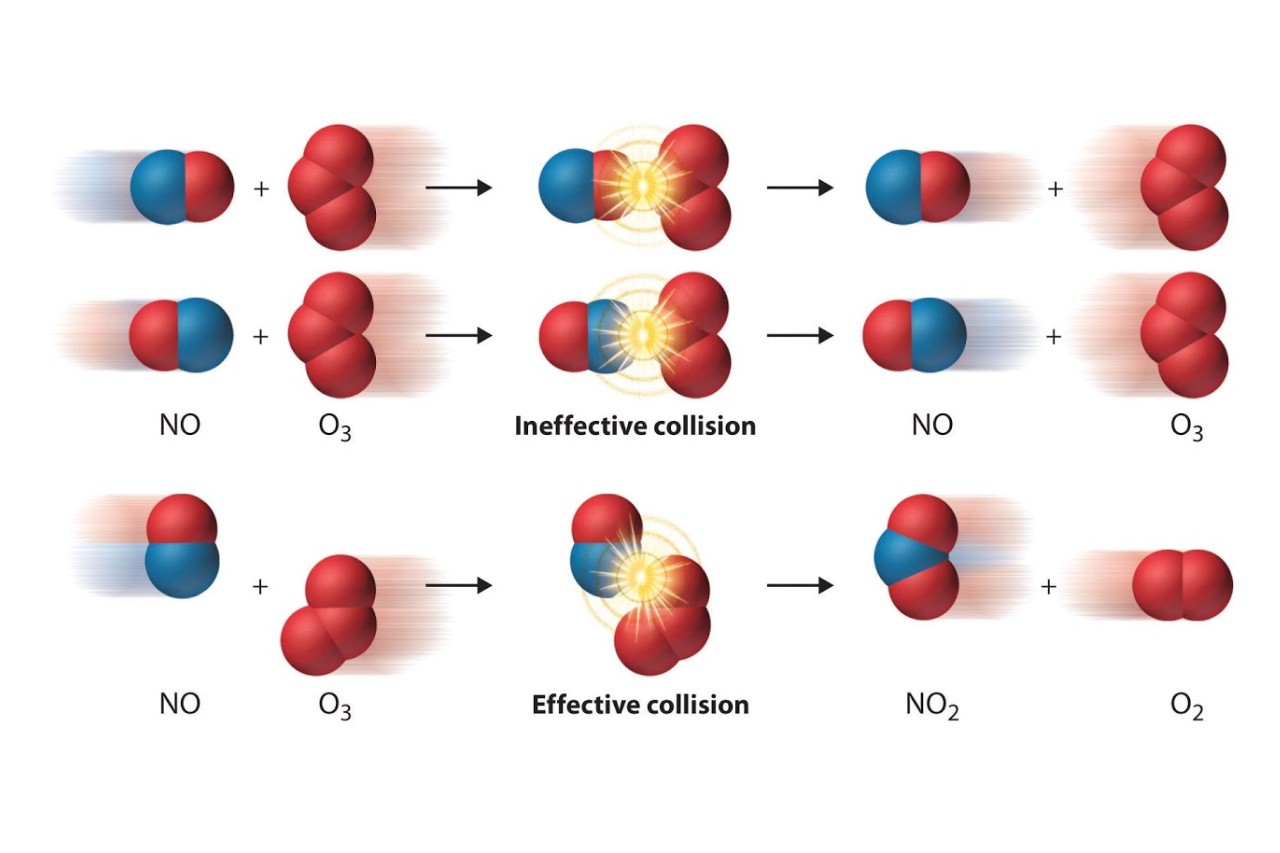
Collision theory also emphasizes the importance of the correct molecular orientation during collisions. In order for a reaction to take place, the colliding molecules must have the correct arrangement of atoms to form new bonds. Incorrect orientation can result in unsuccessful collisions and no reaction.
Validity in Gas Phase Reactions
Collision theory is particularly applicable to gas phase reactions, where molecules are constantly moving and colliding with each other. The theory provides a useful framework for understanding and predicting the behavior of these reactions.
Reaction Cross-Sections
Collision theory introduces the concept of reaction cross-sections, which represent the effective area that molecules need to come into contact with in order to react. The larger the reaction cross-section, the higher the probability of a successful collision and subsequent reaction.
Transition State Theory
Collision theory forms the basis for transition state theory, which provides a more detailed understanding of reaction mechanisms. Transition state theory incorporates the concept of activated complex, a high-energy intermediate state that forms during a chemical reaction.
Applications in Industrial Processes
Collision theory is widely used in industrial processes to optimize reaction conditions and maximize yield. By understanding the factors that affect reaction rates, scientists and engineers can design efficient reaction systems and improve manufacturing processes.
Collision Theory and Reaction Mechanisms
Collision theory helps explain reaction mechanisms by analyzing the intermediate steps that occur during a chemical reaction. It allows us to investigate the role of specific molecules and their interactions, providing valuable insights into the overall reaction process.
Validating Kinetic Models
Collision theory provides a method to validate kinetic models and determine the reaction mechanism. By comparing experimental results with the predictions of collision theory, scientists can confirm the accuracy of their kinetic models and refine their understanding of the reaction kinetics.
The Influence of Pressure
According to collision theory, increasing the pressure on a gas-phase reaction can lead to an increase in the reaction rate. This is because higher pressure increases the number of collisions between molecules, thus increasing the chances of successful collisions.
The Effect of Surface Area
In heterogeneous reactions, where reactants are in different phases (e.g., solid and gas), increasing the surface area of the solid reactant can enhance the reaction rate. By exposing more reactant particles to the gas phase, the frequency of collisions increases, leading to a higher reaction rate.
Deviations from Collision Theory
While collision theory provides a useful framework for understanding chemical reactions, there are instances where it may not fully explain observed reaction behavior. Quantum mechanical effects, solvent effects, and complex reaction mechanisms can sometimes lead to deviations from the predictions of collision theory.
Rate Constant and Collision Frequency
Collision theory states that the rate of a reaction is proportional to the frequency of collisions between reactant molecules. The rate constant, which is specific to a particular reaction, represents the proportionality constant between the collision frequency and the rate of reaction.
Importance in Understanding Biological Reactions
Collision theory is not only relevant to chemical reactions but also plays a crucial role in understanding biological processes. It provides insights into enzyme-substrate interactions, protein folding, and other essential biological reactions.
Advancements in Collision Theory
Over the years, collision theory has been refined and expanded upon through advancements in experimental techniques and computational methods. These developments have allowed scientists to gain a deeper understanding of reaction kinetics and implement collision theory in various fields of study.
Limitations of Collision Theory
While collision theory provides valuable insights into chemical reactions, it does have some limitations. It assumes that all collisions between reactant molecules are effective and that the rate of reaction solely depends on the frequency of collisions, neglecting other factors such as steric effects and specific molecular interactions.
Influence on Chemical Education
Collision theory has had a significant impact on chemical education, shaping the way we teach and learn about chemical reactions. It provides a framework for discussing reaction rates and factors that affect them, helping students grasp fundamental concepts in chemistry.
These 19 astonishing facts about collision theory highlight its fundamental role in understanding the kinetics and mechanisms of chemical reactions. From its origins to its applications in various fields, collision theory continues to shed light on the intricate processes that occur at the molecular level. By studying the principles of collision theory, scientists can unlock new insights into reaction kinetics and pave the way for advancements in chemistry and related disciplines.
Conclusion
In conclusion, the concept of collision theory is a fundamental aspect of chemistry that explains the behavior of chemical reactions. By understanding the factors influencing the rate of reactions and the importance of molecular collisions, scientists can make significant advancements in various fields, including pharmaceuticals, materials science, and environmental studies. Collision theory provides valuable insights into the complex mechanisms that drive chemical reactions and helps unravel the mysteries of molecular interactions.It is crucial to appreciate the astonishing facts about collision theory and recognize its significance in our understanding of the world around us. From the role of activation energy to the impact of molecular orientation, each aspect of collision theory contributes to our knowledge of chemical reactions. By delving deeper into collision theory, scientists open up endless possibilities for innovation and discovery.
FAQs
1. What is collision theory?
Collision theory is a concept in chemistry that explains how chemical reactions occur. It states that for a reaction to take place, reactant molecules must collide with enough energy and proper orientation.
2. Who proposed collision theory?
Collision theory was first proposed by Max Trautz and William Lewis in the early 20th century.
3. What factors affect the rate of a chemical reaction according to collision theory?
The factors that affect the rate of a chemical reaction according to collision theory are temperature, concentration, surface area, and the presence of catalysts.
4. Why is activation energy important in collision theory?
Activation energy is the minimum energy required for a reaction to occur. It is essential because it determines the number of reactant molecules with sufficient energy to overcome the energy barrier and proceed with the reaction.
5. How does molecular orientation influence the rate of a reaction?
Molecular orientation plays a crucial role in collision theory. For a reaction to occur, reactant molecules must collide in a specific orientation that allows the necessary rearrangement of bonds to form new products.
6. Can collision theory be applied to all chemical reactions?
No, collision theory may not apply to all chemical reactions. Some complex reactions involve intermediate steps, and factors other than collisions may come into play.
7. How does collision theory contribute to the field of pharmaceuticals?
Collision theory helps pharmaceutical researchers understand how drugs interact with target molecules in the body. By studying reaction rates and optimizing conditions, scientists can design more effective medications.
8. What is the role of catalysts in collision theory?
Catalysts increase the rate of a reaction by providing an alternative pathway with lower activation energy. This lowers the energy barrier required for reactant molecules to collide effectively.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
