The concept of covalent radius is a fundamental aspect of chemistry that plays a crucial role in understanding the characteristics of chemical bonds and the behavior of many elements and compounds. Covalent radius refers to the size of an atom when it forms a covalent bond with another atom. It provides valuable insights into the arrangement of electrons and the overall structure of molecules.
In this article, we will explore 19 fascinating facts about covalent radius that will expand your knowledge of this important concept. From the origin of the term to its variations across the periodic table, these facts will shed light on the intricacies of covalent radius and its impact on chemical interactions. Whether you’re a chemistry enthusiast, a student, or simply curious about the wonders of the molecular world, get ready to dive into the captivating world of covalent radius!
Key Takeaways:
- Covalent radius determines the size of atoms when they bond, affecting bond strength and molecular shape. It’s like the personal space between atoms, influencing how they interact and form molecules.
- Understanding covalent radius helps scientists predict how elements behave in reactions and design new materials. It’s like having a secret code to unlock the mysteries of chemistry and create amazing technologies.
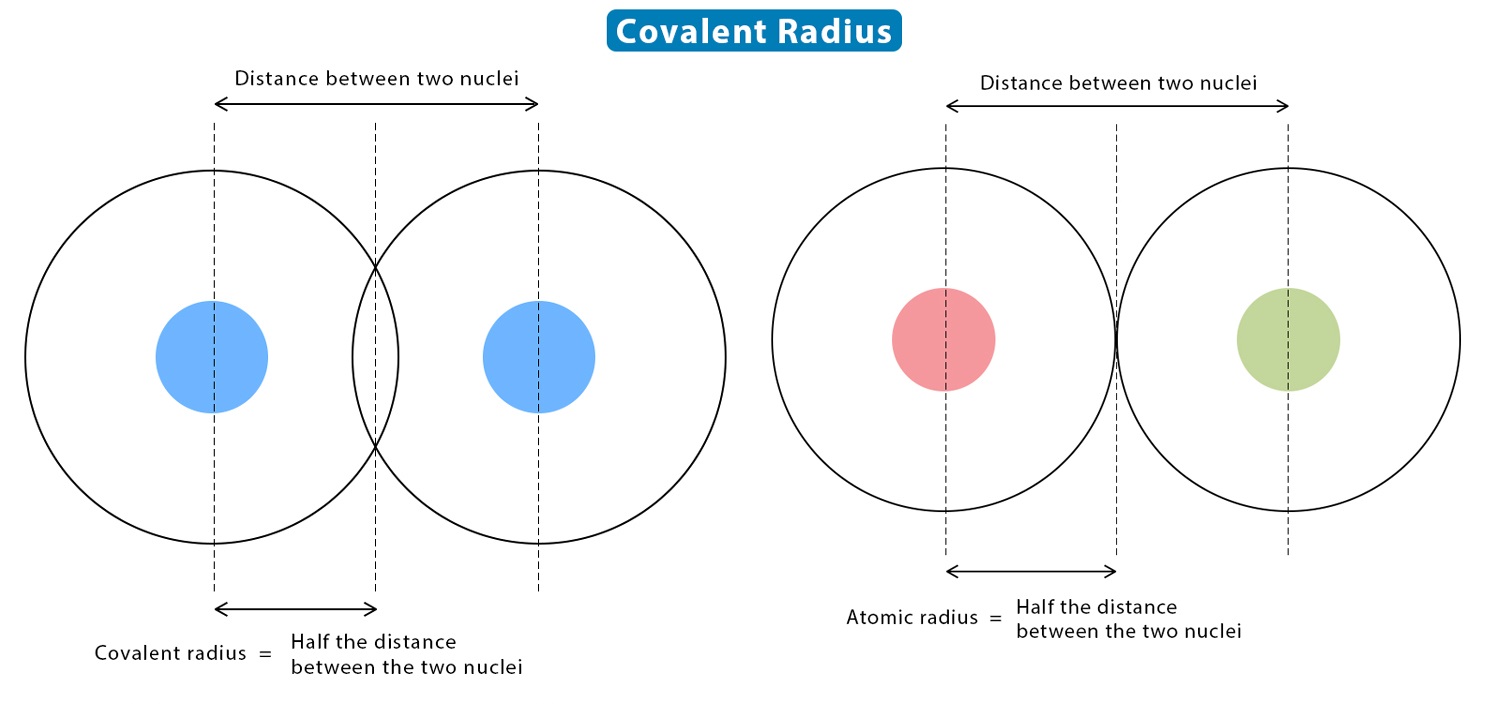
Covalent Radius Explained
The covalent radius is a measure of the size of an atom when it forms a covalent bond with other atoms. It represents the distance between the atomic nucleus and the outermost electron shell.
Relationship to Bonding
The covalent radius determines the strength and nature of chemical bonds. As the covalent radius increases, the bond length typically increases as well.
Ionic Radius vs. Covalent Radius
The covalent radius is different from the ionic radius, which refers to the size of an ion. While both are measures of atomic size, the covalent radius is generally smaller than the ionic radius.
Variation Across the Periodic Table
The covalent radius of an element tends to decrease across a period in the periodic table from left to right. This trend is primarily influenced by the increasing nuclear charge and the attraction between the nucleus and the electrons.
Group Trends
Within a group in the periodic table, the covalent radius generally increases as you move down the group. This is due to the addition of new electron shells, which increases the distance between the nucleus and the outermost electrons.
Noble Gases and Covalent Radius
Noble gases have the smallest covalent radii among all the elements. This is because they have a full complement of electrons in their outermost shell, making them stable and less likely to form covalent bonds.
Transition Metals and Covalent Radius
Transition metals often have variable covalent radii due to their ability to form multiple oxidation states. The covalent radius can change depending on the oxidation state of the metal.
Covalent Radius and Bond Strength
In general, a smaller covalent radius corresponds to a stronger bond between atoms. This is because the electrons are held more tightly by the nucleus, resulting in a greater sharing of electron density.
Covalent Radius and Molecular Shape
The covalent radius of an atom influences the overall shape of a molecule. Different covalent radii can lead to variations in bond angles and molecular geometry.
Covalent Radius and Chemical Reactivity
The covalent radius can also affect an atom’s reactivity. Atoms with larger covalent radii tend to be more reactive as they have a greater ability to interact with other atoms and form bonds.
Covalent Radius and Van der Waals Radius
The van der Waals radius is a measure of the size of an atom in a non-bonded state. In general, the van der Waals radius is larger than the covalent radius.
Covalent Radius and Periodic Trends
Covalent radii are part of the larger periodic trend that governs the physical and chemical properties of elements. Understanding these trends can provide insights into the behavior of elements in various chemical reactions.
Covalent Radius and Electronegativity
There is a correlation between the covalent radius of an element and its electronegativity, which is a measure of its ability to attract shared electrons. Generally, elements with smaller covalent radii tend to have higher electronegativities.
Measurement of Covalent Radius
Covalent radii can be determined through experimental methods such as X-ray crystallography and spectroscopy. These techniques allow scientists to visualize the arrangement of atoms in a molecule and measure their distances.
Applications in Materials Science
Understanding covalent radius is crucial in materials science and engineering. It helps in predicting the physical and chemical properties of materials, designing new compounds, and developing advanced technologies.
Covalent Radius and Bond Energy
The covalent radius has a direct impact on the bond energy between atoms. A smaller covalent radius results in stronger bonds, requiring more energy to break.
Covalent Radius and Periodic Table Trends
The periodic table provides a valuable framework to analyze trends in covalent radii across elements. These trends help chemists make predictions about the behavior of elements in various chemical reactions.
Covalent Radius in Organic Chemistry
Covalent radii are crucial in organic chemistry, where understanding the size and shape of atoms is essential for predicting reaction outcomes and designing new compounds.
Covalent Radius and Molecular Interactions
The covalent radius plays a significant role in determining the strength of molecular interactions, such as hydrogen bonding, van der Waals forces, and dipole-dipole interactions.
Conclusion
In conclusion, understanding the concept of covalent radius is crucial in the study of chemistry. Covalent radius plays a significant role in determining the size and properties of atoms and molecules. It provides valuable information about the bond length, bond strength, and overall stability of chemical compounds.
By considering the various factors that influence covalent radius, such as atomic number, atomic size, and electron-electron repulsion, scientists are able to predict and explain many chemical phenomena. The covalent radius also serves as a useful tool in designing and synthesizing new materials with specific properties.
Exploring the 19 fascinating facts about covalent radius discussed in this article showcases the immense complexity and beauty of the chemical world. As scientists continue to unravel the mysteries of covalent radius and its implications, the applications and discoveries in chemistry will undoubtedly expand.
FAQs
1. What exactly is covalent radius?
Covalent radius refers to the distance between the atomic nucleus and the outermost electron in a covalent bond. It is a measure of the size of an atom when it forms a covalent bond.
2. How is covalent radius determined?
The covalent radius can be derived from experimental measurements, such as X-ray diffraction or spectroscopy. It can also be theoretically calculated using quantum mechanical calculations.
3. What factors influence the covalent radius?
The covalent radius is influenced by various factors, including the atomic number of the atom, the electronic configuration, atomic size, and electron-electron repulsion.
4. How does the covalent radius affect chemical properties?
The covalent radius affects many chemical properties, such as bond length, bond strength, and overall stability of chemical compounds. It can also influence the reactivity and polarity of molecules.
5. Can covalent radius be used to predict the behavior of elements?
Absolutely! The covalent radius provides valuable insights into the behavior of elements and their ability to form bonds and interact with other atoms. It helps in predicting the nature of chemical reactions and the formation of compounds.
6. Are there any exceptions or limitations to covalent radius?
While covalent radius is a useful concept, it is important to note that it is an average value and may vary depending on the specific molecule or bond type. The presence of multiple bonding partners or complex molecular structures can lead to deviations from the predicted covalent radius values.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
