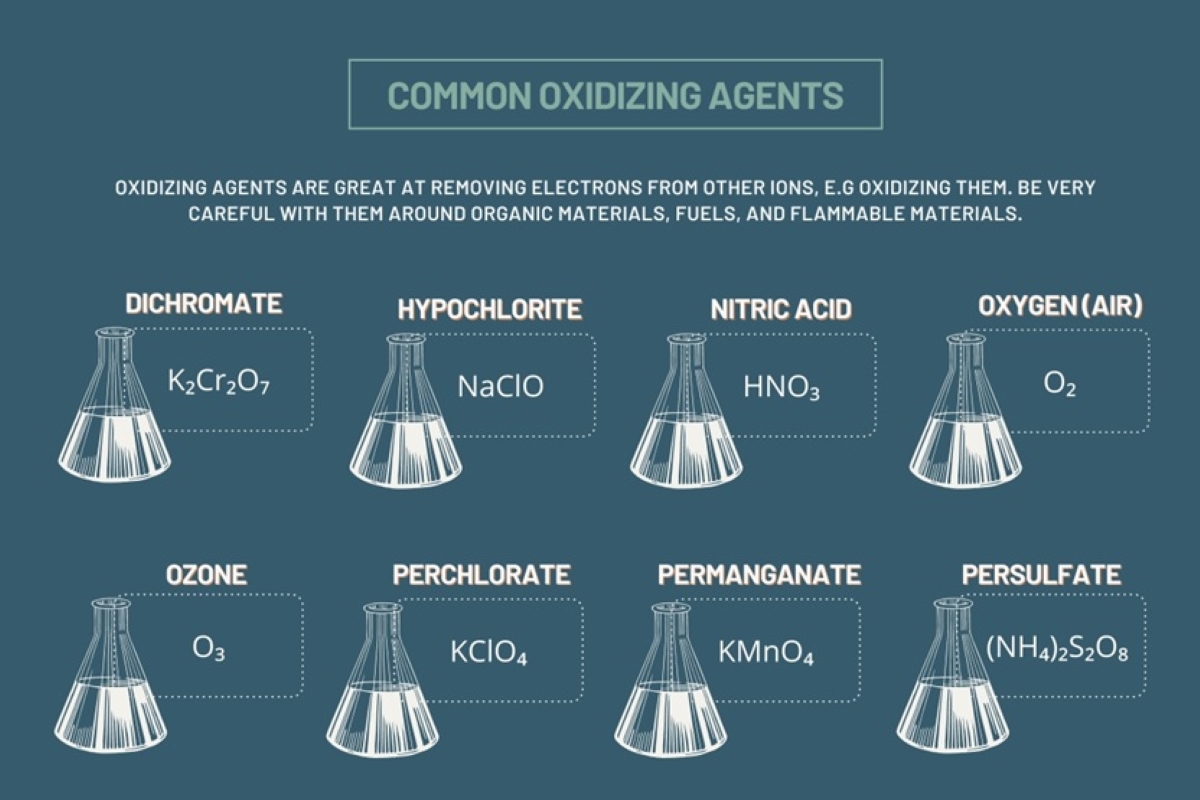
When it comes to understanding chemistry, one of the most intriguing areas of study involves oxidizing agents. These compounds play a crucial role in various chemical reactions, making them an essential part of our everyday lives. Oxidizing agents are substances that have the ability to accept electrons from other compounds, resulting in the oxidation of those substances.
In this article, we will explore 8 enigmatic facts about oxidizing agents that will expand your knowledge of this fascinating field. From their role in combustion reactions to their use in the pharmaceutical industry, oxidizing agents have a wide range of applications and implications. So, if you’re ready to delve into the world of oxidizing agents and unravel their mysteries, let’s dive in and discover the fascinating facts that make them so intriguing!
Key Takeaways:
- Oxidizing agents play a crucial role in chemical reactions, including combustion and rust formation, and can be found in everyday products like bleach and hydrogen peroxide.
- Understanding the properties of oxidizing agents helps us use their potential in chemistry and everyday life, while also being aware of their potential hazards on the environment.
Essential Role in Chemical Reactions
An oxidizing agent is a substance that facilitates oxidation reactions by accepting electrons from other substances. It plays a crucial role in various chemical reactions, including combustion, synthesis, and corrosion.
Oxidizing Agents in Everyday Life
Oxidizing agents can be found in numerous everyday products, such as bleach, hydrogen peroxide, and even in the air we breathe. They are used in cleaning agents, hair dyes, and water treatment processes.
Powerful Oxidizing Agents
Sodium hypochlorite (NaOCl) and potassium permanganate (KMnO4) are renowned for their strong oxidizing properties. They are commonly used in laboratory experiments and industrial applications.
Oxidizing Agents and Fire
Oxidizing agents are often associated with fire hazards. Substances like oxygen, fluorine, and chlorine can support the combustion process by providing the necessary oxygen molecules for burning.
Oxidizing Agents and Rust
Rust formation on metals is a common result of oxidation reactions. Oxidizing agents, such as oxygen and moisture in the air, react with iron or other metals to form the characteristic reddish-brown coating we know as rust.
Oxidizing Agents in Organic Chemistry
Oxidizing agents are extensively used in organic chemistry to introduce functional groups or convert one compound into another. Potassium dichromate (K2Cr2O7) and potassium permanganate (KMnO4) are often employed for this purpose.
Oxidizing Agents and Biology
In biological systems, oxidizing agents play vital roles. The respiratory chain in mitochondria involves a series of oxidation reactions to produce energy in the form of adenosine triphosphate (ATP).
Oxidizing Agents in Environmental Impact
Industrial activities and human consumption of oxidizing agents can have environmental implications. Some oxidizing agents contribute to air pollution, and improper disposal can contaminate water sources and soil.
In conclusion, these 8 enigmatic facts about oxidizing agents shed light on their significance and influence in various fields of science and everyday life. Understanding the properties and behavior of oxidizing agents helps us utilize their potential while also being aware of their potential hazards.
Conclusion
Oxidizing agents play a crucial role in various chemical processes, and their enigmatic nature continues to intrigue scientists and researchers. Through this article, we have explored eight fascinating facts about oxidizing agents that shed light on their importance and impact. From their ability to accept electrons to their involvement in redox reactions, oxidizing agents are key players in the world of chemistry.
Understanding the properties and effects of oxidizing agents is essential for both students and professionals in the field of chemistry. By continuously studying and researching these compounds, we can unlock new discoveries and applications in various industries, from pharmaceuticals to environmental science.
So, the next time you encounter an oxidizing agent, remember its potential to drive chemical reactions and its ability to bring about remarkable transformations in the world of chemistry.
FAQs
Q: What is an oxidizing agent?
An oxidizing agent is a substance that is capable of accepting electrons from other substances during a chemical reaction, effectively causing oxidation.
Q: What are some common examples of oxidizing agents?
Common examples of oxidizing agents include oxygen, hydrogen peroxide, chlorine, and potassium permanganate.
Q: How do oxidizing agents work?
Oxidizing agents work by undergoing reduction themselves, where they gain electrons, while causing oxidation in the substances they react with.
Q: What are the uses of oxidizing agents?
Oxidizing agents have numerous applications, such as in the production of chemicals, bleaching processes, disinfectants, and rocket propellants.
Q: Are oxidizing agents dangerous?
Some oxidizing agents can be hazardous and reactive, making them potentially dangerous if mishandled. It is important to follow proper safety precautions when working with these substances.
Q: Can oxidizing agents be found in everyday products?
Yes, oxidizing agents can be found in everyday products such as household cleaning agents, hair dyes, and toothpaste.
Q: What happens when an oxidizing agent reacts with a reducing agent?
When an oxidizing agent reacts with a reducing agent, it initiates a redox reaction, where the oxidizing agent gains electrons (reduction) and the reducing agent loses electrons (oxidation).
Q: Can oxidizing agents be used for environmental purposes?
Yes, oxidizing agents can be used in environmental applications such as water treatment and pollution control, where they can facilitate the breakdown of harmful substances.
Oxidizing agents play a crucial role in our world, from everyday life to complex chemical processes. If you're curious to learn more, explore the fascinating world of redox potential and electron transfer in our article "15 Astounding Facts About Redox Potential." For a deeper understanding of how oxidizing agents work, check out "14 Captivating Facts About Reaction Coordinate," which delves into the intricacies of chemical reactions. And if you're eager to discover the full scope of oxidation-reduction processes, don't miss "19 Astonishing Facts About Redox Reaction," where we uncover the secrets of redox reactions.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


