The field of chemistry is vast and intricate, encompassing a wide range of reactions and processes that contribute to our understanding of the world around us. One such reaction that has captured the attention of chemists and researchers is the elimination reaction. This fascinating process involves the removal of atoms or functional groups from a molecule, leading to the formation of a new double bond or the creation of a new molecule altogether. Elimination reactions are essential in various chemical industries, including pharmaceuticals, polymers, and materials science. From their role in organic synthesis to their significance in biochemical pathways, these reactions have numerous applications and implications. In this article, we will explore 19 intriguing facts about elimination reactions, providing a deeper insight into this captivating aspect of chemistry. So, let’s dive in and uncover the secrets behind these transformative reactions.
Key Takeaways:
- Elimination reactions are like chemical makeovers, where molecules lose parts to create new products. They’re important in making medicines and even plastics, and they help chemists design better and greener processes.
- Just like puzzle pieces fitting together, elimination reactions help chemists create new bonds and molecules. They’re like the magic behind making new and exciting things in the world of chemistry!
Definition of Elimination Reaction
The elimination reaction is a chemical process in which a molecule loses atoms or groups of atoms, resulting in the formation of a new product. It involves the removal of small molecules like water, hydrogen halides, or carbon dioxide from a larger molecule, resulting in the formation of multiple products.
Types of Elimination Reactions
There are two main types of elimination reactions: E1 and EThe E1 (unimolecular elimination) reaction involves a two-step mechanism, while the E2 (bimolecular elimination) reaction occurs in a single step. These reactions play a key role in organic chemistry, allowing for the synthesis of new compounds and the formation of double or triple bonds.
Importance of Elimination Reactions in Pharmaceuticals
Elimination reactions are crucial in the pharmaceutical industry as they enable the production of drugs and therapeutic agents. The ability to selectively remove certain functional groups from molecules is essential for modifying drug structures, improving their potency, and reducing side effects.
Common Examples of Elimination Reactions
Some common examples of elimination reactions include the dehydration of alcohols to form alkenes, the dehydrohalogenation of alkyl halides to form alkenes, and the decarboxylation of carboxylic acids to form alkenes or alkynes.
Role of Catalysts in Elimination Reactions
Catalysts can greatly enhance the rate of elimination reactions by lowering the activation energy required for the reaction to occur. For example, strong bases such as hydroxide ions (OH-) or alkoxides (RO-) can act as catalysts in E2 reactions.
Regioselectivity in Elimination Reactions
Regioselectivity refers to the preference of elimination reactions to occur at specific positions within a molecule. Factors such as the stability of the resulting products, the nature of the substituents, and the presence of functional groups can influence the regioselectivity of the reaction.
Stereoselectivity in Elimination Reactions
Stereoselectivity refers to the preference of elimination reactions to form specific stereoisomeric products. Depending on the reaction conditions and the geometry of the reacting molecules, either the E (trans) or Z (cis) isomer may be favored.
The Saytzeff’s Rule
Saytzeff’s rule states that in elimination reactions, the major product is usually the one formed by the removal of the ?-hydrogen (hydrogen on the carbon adjacent to the carbon bearing the leaving group) that gives rise to the most substituted alkene. This is due to the stability conferred by the increased number of substituents on the double bond.
Industrial Applications of Elimination Reactions
Elimination reactions find numerous applications in the industry, such as the production of plastics, synthetic fibers, and biofuels. These reactions allow for the efficient formation of complex organic molecules on a large scale.
The E1 Reaction Mechanism
The E1 mechanism involves a stepwise process where the leaving group departs, forming a carbocation intermediate. The carbocation then loses a proton, resulting in the formation of the double bond.
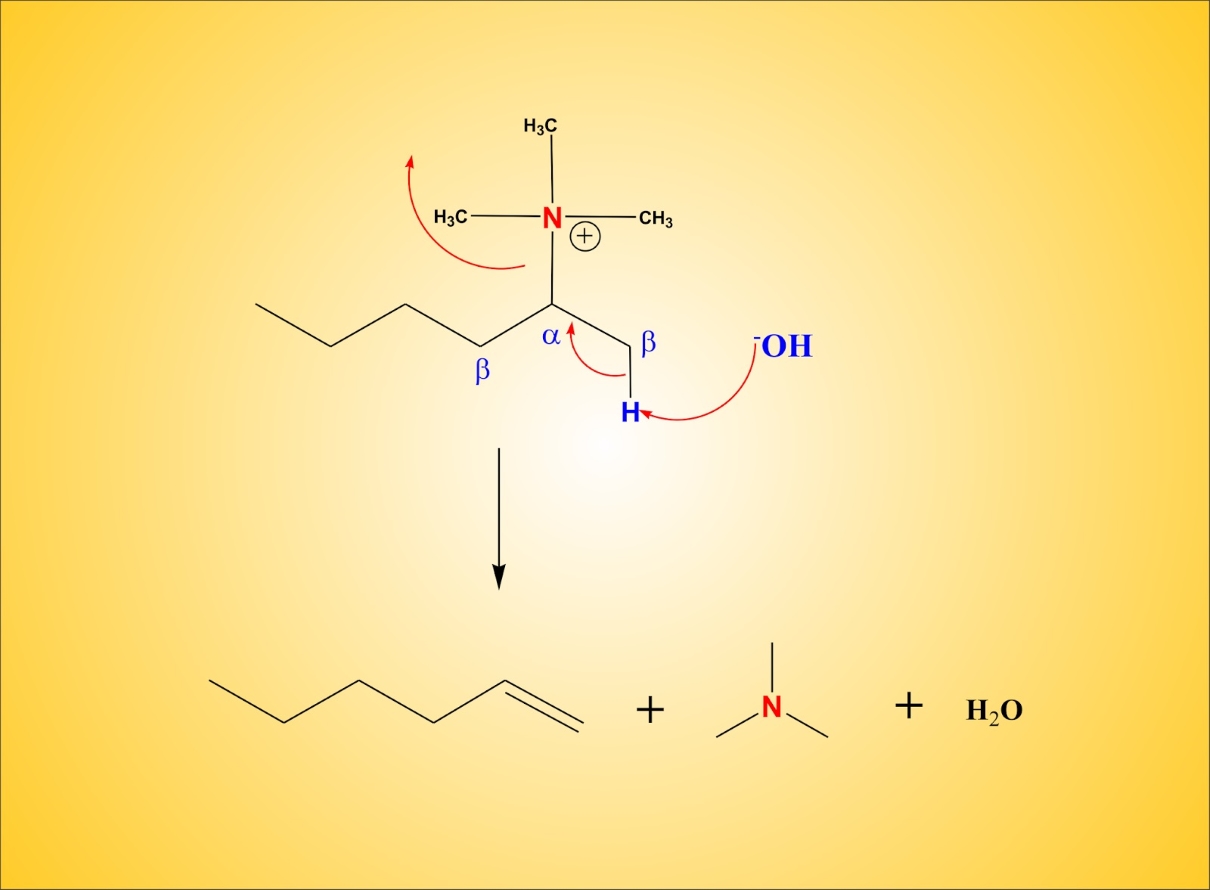
The E2 Reaction Mechanism
The E2 mechanism is a concerted process where the leaving group and a proton are eliminated simultaneously. This leads to the formation of the double bond and the expulsion of the leaving group.
Factors Influencing the Rate of Elimination Reactions
The rate of elimination reactions can be influenced by several factors, including the concentration of reactants, temperature, solvent, and steric hindrance around the reaction site.
Comparison of Elimination and Substitution Reactions
Elimination reactions differ from substitution reactions in that elimination involves the removal of atoms or groups to form a new product, while substitution replaces one atom or group with another. Both types of reactions can occur, depending on the reaction conditions and the nature of the reactants.
Biological Significance of Elimination Reactions
Within living organisms, elimination reactions play a vital role in various biochemical processes. For example, the elimination of water during protein synthesis facilitates the formation of peptide bonds between amino acids.
Carbon-Carbon Bond Formation in Elimination Reactions
Elimination reactions are often utilized in carbon-carbon bond formation reactions, such as the Heck reaction, which enables the synthesis of complex organic molecules with diverse structures.
The Effect of Temperature on Elimination Reactions
Increasing the temperature can accelerate elimination reactions by providing greater kinetic energy to the reactant particles, leading to more frequent and energetic collisions that favor the breaking of bonds and formation of the double bond.
Elimination Reactions in Alcohol Dehydration
Alcohol dehydration is a common elimination reaction that produces alkenes. By removing a water molecule from an alcohol molecule, the double bond is formed, which allows for the synthesis of various organic compounds.
The Substrate Structure in Elimination Reactions
The structure of the substrate can have a significant impact on the rate and selectivity of elimination reactions. Substrates with bulky groups or hindered positions may hinder the reaction or favor alternative pathways.
The Importance of Understanding Elimination Reactions
Understanding elimination reactions is crucial for synthetic chemists, as they provide valuable tools for the creation of complex organic compounds and the design of more efficient and sustainable chemical processes.
Conclusion
Elimination reactions are a fascinating area of study in the field of chemistry. They play a crucial role in various chemical processes and have significant applications in both academic research and industrial production. From understanding the mechanism behind elimination reactions to exploring their diverse applications, there is plenty to discover and appreciate about this fundamental concept.
By delving into the intricacies of elimination reactions, chemists can uncover valuable insights into the reactivity of different compounds and design more efficient synthesis routes for various organic compounds. The ability to control and manipulate elimination reactions opens up new possibilities for drug discovery, material synthesis, and the development of sustainable chemical processes.
Whether you are a chemistry enthusiast or a professional in the field, exploring the world of elimination reactions can be both intellectually stimulating and practically rewarding. By understanding the underlying principles and applications of these reactions, you can make meaningful contributions to the advancement of chemistry and its diverse applications in our world.
FAQs
1. What is an elimination reaction?
An elimination reaction is a type of chemical reaction in which a molecule loses atoms or groups of atoms to form a new compound. It is characterized by the removal of functional groups, typically leading to the formation of a double bond or a ring structure.
2. What is the difference between elimination and substitution reactions?
In an elimination reaction, a molecule loses atoms or groups of atoms, resulting in the formation of a double bond or a ring structure. In contrast, a substitution reaction involves the replacement of one functional group with another without any change in the number of bonds.
3. What are some common examples of elimination reactions?
Some common examples of elimination reactions include the dehydration of alcohols, the dehydrohalogenation of alkyl halides, and the decarboxylation of carboxylic acids.
4. How are elimination reactions important in organic synthesis?
Elimination reactions are key steps in organic synthesis as they enable the formation of complex organic molecules. By selectively removing certain functional groups, chemists can create new compounds with specific properties and functionalities.
5. Are elimination reactions reversible?
Elimination reactions can be reversible under certain conditions, particularly if the reactants or products are thermodynamically unstable. However, in many cases, elimination reactions are irreversible due to the high energy barriers associated with reversing the reaction.
Elimination reactions are a fascinating aspect of chemistry, but there's so much more to explore in this captivating field. Uncover the secrets of organic chemistry, from the building blocks of life to the complex reactions that shape our universe. And if you're curious about the intricate dance of molecules, take a closer look at how reaction coordinates guide chemical transformations every step of the way.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
