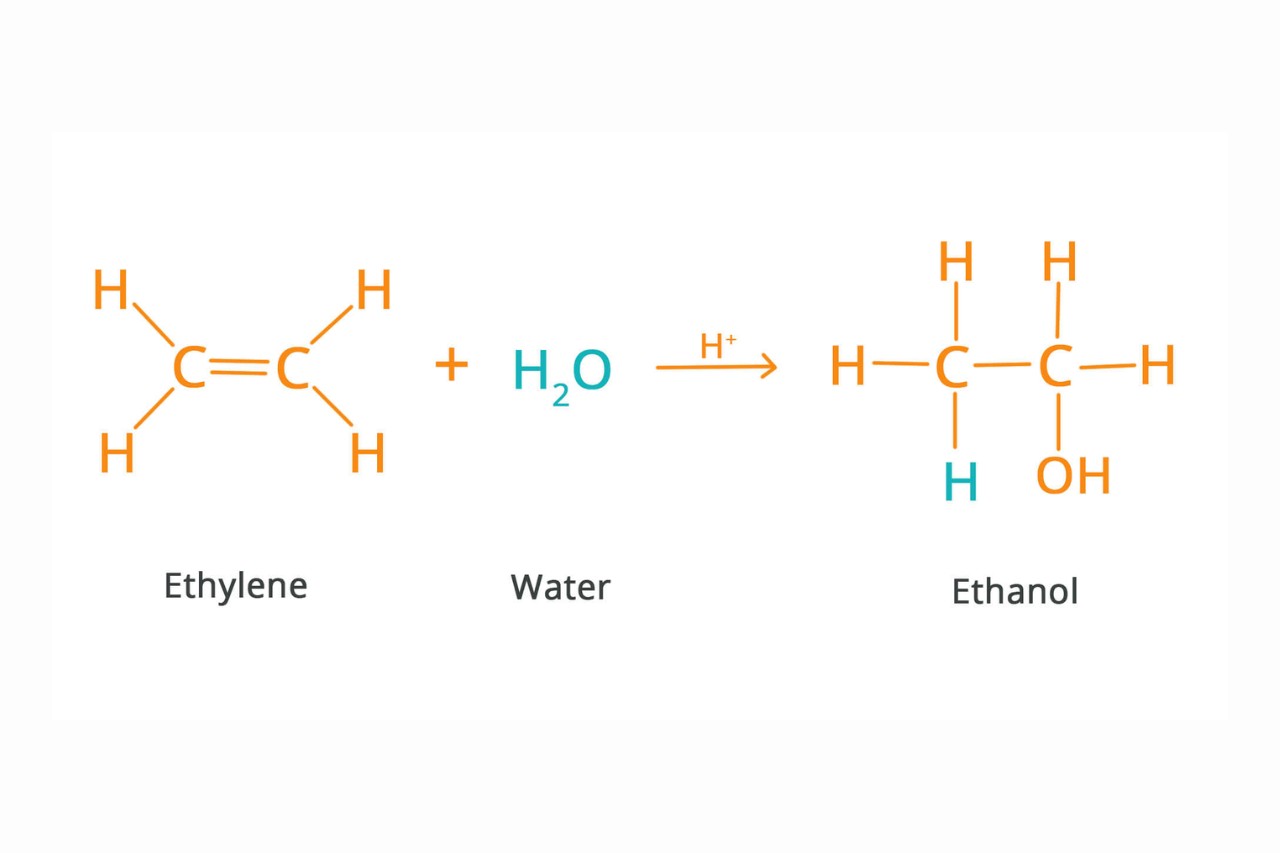
Addition reaction, also known as a direct or simple reaction, is a fundamental concept in organic chemistry that plays a crucial role in understanding chemical reactions. It involves the formation of a new compound by the addition of two or more atoms or groups to a molecule. Addition reactions are not only fascinating but also have significant practical applications in various fields, including pharmaceuticals, materials science, and biochemistry.
In this article, we will explore 10 extraordinary facts about addition reactions that will deepen your understanding of this important chemical process. From the mechanism behind addition reactions to the diverse range of reactions they can undergo, these facts will fascinate both chemistry enthusiasts and professionals in the field. So, let’s embark on this journey to uncover the intriguing world of addition reactions!
Key Takeaways:
- Addition reactions combine substances to create new products, influencing the properties and reactivity of compounds in chemistry.
- Addition reactions are crucial in creating materials like plastics, synthesizing pharmaceuticals, and shaping the field of organic chemistry.
Addition reactions involve the combination of two or more substances.
Addition reactions are a type of chemical reaction where two or more substances combine to form a single product. This process is characterized by the breaking of multiple chemical bonds and the formation of new ones.
Addition reactions are commonly observed in organic chemistry.
Organic chemistry focuses on the study of carbon-based compounds, and addition reactions play a fundamental role in this field. They are used to synthesize new organic compounds and create complex molecular structures.
Addition reactions can be classified as electrophilic or nucleophilic.
In electrophilic addition reactions, an electrophile (electron-deficient species) reacts with a nucleophile (electron-rich species). In nucleophilic addition reactions, a nucleophile attacks and bonds with an electrophile.
Addition reactions often result in the formation of new functional groups.
Functional groups are specific groups of atoms within a molecule that determine its chemical behavior. Addition reactions are capable of introducing new functional groups into a compound, altering its properties and reactivity.
Addition reactions are highly influenced by reaction conditions.
Variables such as temperature, pressure, solvent, and catalysts can significantly affect the outcome of an addition reaction. Fine-tuning these conditions allows chemists to control the selectivity and efficiency of the reaction.
Addition reactions play a crucial role in polymerization processes.
Polymerization is the process of combining small monomers to form large polymer chains. Addition reactions, specifically known as addition polymerizations, are responsible for the creation of a wide range of materials, including plastics and synthetic fibers.
Addition reactions can be reversible or irreversible.
In reversible addition reactions, the products formed can react together to reform the original compounds. In irreversible addition reactions, the products are stable and cannot easily revert to the original reactants.
Addition reactions can occur through various mechanisms.
Common mechanisms include radical addition, concerted addition, and stepwise addition. Each mechanism involves distinct steps and intermediates, leading to different reaction outcomes.
Addition reactions often exhibit stereoselectivity.
Stereoselectivity refers to the preference for the formation of a specific stereoisomer during a reaction. Addition reactions can yield different stereoisomers based on the orientation of the reactants and the reaction conditions.
Addition reactions are essential in the synthesis of pharmaceuticals and agrochemicals.
Many drugs and agricultural chemicals are synthesized through addition reactions. This enables chemists to introduce specific functional groups and fine-tune the properties of the resulting compounds for desired biological activities.
The “10 Extraordinary Facts About Addition Reaction” showcases the wide-ranging significance of addition reactions in chemistry. Whether it’s the creation of new functional groups, the synthesis of complex organic compounds, or the production of polymers, addition reactions play a vital role in shaping the field of chemistry. Their versatility and ability to manipulate molecular structures make them a cornerstone of modern chemical synthesis.
So the next time you encounter addition reactions in your studies or research, appreciate the intricacy and importance of these fascinating chemical transformations.
Conclusion
In conclusion, addition reactions are an essential concept in chemistry. They involve the combination of two or more substances to form a new compound. These reactions occur in various chemical processes and have significant applications in industries such as pharmaceuticals, polymers, and organic synthesis.
Understanding the extraordinary facts about addition reactions can deepen your knowledge and appreciation for the intricacies of this important chemical process. From the versatility of the reaction to the application in organic chemistry, addition reactions play a crucial role in advancing scientific exploration and improving our daily lives.
By familiarizing yourself with the fundamental principles of addition reactions, you can gain a deeper understanding of the chemical world and discover the potential for groundbreaking discoveries.
FAQs
1. What is an addition reaction?
An addition reaction is a chemical process in which two or more substances combine to form a new compound. It involves the breaking of existing chemical bonds and the formation of new ones.
2. What are some examples of addition reactions?
Examples of addition reactions include the reaction of hydrogen gas with chlorine gas to form hydrogen chloride, the reaction of alkenes with hydrogen to form alkanes, and the reaction of alkenes with halogens to form alkyl halides.
3. What is the importance of addition reactions?
Addition reactions are vital in various fields, such as organic chemistry, pharmaceuticals, and polymer synthesis. They allow for the creation of new compounds with different properties and functionalities, leading to the development of new materials and drugs.
4. Are all addition reactions reversible?
No, not all addition reactions are reversible. Some addition reactions are irreversible, meaning once the new compound is formed, it cannot easily convert back to its original forms.
5. How are addition reactions different from substitution reactions?
Addition reactions involve the combination of two or more substances, resulting in the formation of a new compound. On the other hand, substitution reactions involve the replacement of one or more atoms or groups with another atom or group.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
