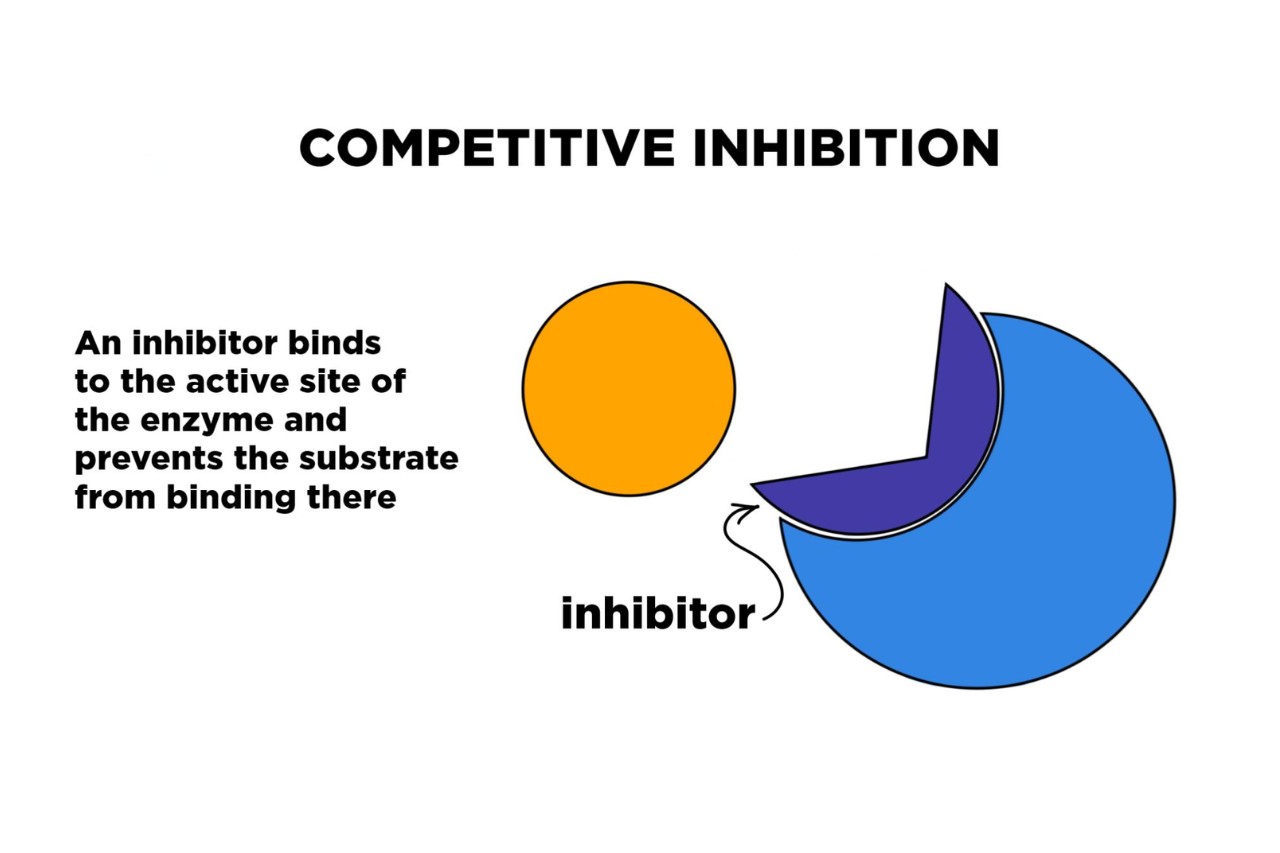
Competitive inhibition is a fascinating concept within the realm of chemistry and biochemistry. It is a phenomenon that occurs when a molecule, known as the inhibitor, competes with the substrate for the active site of an enzyme, effectively reducing or blocking the enzyme’s activity. Competitive inhibition plays a crucial role in various biochemical processes and has significant implications in fields such as pharmacology and drug design. In this article, we will explore 10 extraordinary facts about competitive inhibition that will enhance your understanding of this essential concept. From its impact on enzyme kinetics to its role in drug resistance, competitive inhibition offers a wealth of intriguing insights into the intricate workings of chemical reactions. So, let’s dive into the extraordinary world of competitive inhibition!
Key Takeaways:
- Competitive inhibition is when a substance blocks an enzyme’s activity by competing with the natural substrate. It’s like a game of musical chairs at the enzyme’s active site!
- Understanding competitive inhibition helps scientists develop drugs and regulate enzyme activity, leading to potential breakthroughs in medicine and biochemistry. It’s like unlocking a secret code to treat diseases!
Competitive Inhibition is a Common Phenomenon
Competitive inhibition is a widely observed phenomenon in the field of biochemistry and enzymology. It occurs when a substance, known as the inhibitor, competes with the natural substrate for the active site of an enzyme. This competition prevents the substrate from binding to the enzyme, thereby inhibiting its activity.
It Can Affect Enzyme Function
When a competitive inhibitor binds to the active site of an enzyme, it prevents the substrate from binding, thus reducing or completely stopping the enzymatic reaction. This inhibition can have a significant impact on various biological processes and pathways, ranging from metabolism to cell signaling.
Competitive Inhibitors Have Similar Structures to Substrates
One fascinating aspect of competitive inhibition is that the inhibitors often have structural similarities to the natural substrate. This similarity allows them to bind to the active site of the enzyme and compete for the same binding sites.
Affinity Determines the Strength of Competitive Inhibition
The strength of competitive inhibition depends on the affinity of the inhibitor towards the active site of the enzyme. Higher affinity inhibitors will be able to outcompete the substrate more effectively, resulting in a stronger inhibition of enzyme activity.
Competitive Inhibition Can Be Reversible
Unlike other forms of enzyme inhibition, competitive inhibition is often reversible. This means that once the inhibitor is removed or diluted, the enzyme can regain its normal activity by binding to the substrate without competition.
Competitive Inhibition Can Be Overcome by Increasing Substrate Concentration
One way to overcome competitive inhibition is by increasing the concentration of the substrate. By providing a higher concentration of the natural substrate, it becomes more likely to bind to the enzyme and outcompete the inhibitor.
Competitive Inhibition Can Help Regulate Enzyme Activity
In some cases, competitive inhibition plays a crucial role in regulating enzymatic activity. By having specific inhibitors compete with substrates, the cell can fine-tune the activity of key enzymes in response to changing conditions or metabolic demands.
Competitive Inhibition Can Be Pharmacologically Exploited
The understanding of competitive inhibition has led to the development of pharmacological interventions. Drugs can be designed as competitive inhibitors to target specific enzymes involved in disease pathways, offering a potential strategy for therapeutic intervention.
Competitive Inhibition Studies Can Aid Drug Discovery
Studying competitive inhibition can provide valuable insights into enzyme-substrate interactions. This knowledge can be utilized in the process of drug discovery and developing new molecules that can act as competitive inhibitors for specific enzymes.
Competitive Inhibition is an Active Area of Research
Competitive inhibition continues to be an active field of research. Scientists are constantly exploring new inhibitors, investigating the structures of enzyme-inhibitor complexes, and studying the implications of competitive inhibition in various biological processes.
Conclusion
In conclusion, competitive inhibition is a fascinating aspect of enzymology that plays a crucial role in various biochemical processes. The extraordinary facts about competitive inhibition shed light on the intricate mechanisms by which enzymes function and are regulated. From the concept of reversible binding to the impact on enzyme kinetics and the potential applications in drug development, competitive inhibition provides valuable insights into the complex world of enzymatic reactions.Understanding the dynamics of competitive inhibition not only contributes to our fundamental knowledge of biochemistry but also holds immense potential for practical applications. By manipulating competitive inhibitors, scientists can develop strategies to modulate enzyme activity, leading to the development of new therapies and treatments for various diseases and disorders.As research in the field of competitive inhibition continues to progress, we can expect to uncover even more extraordinary facts and delve deeper into the intricate details of enzymatic regulation. This knowledge will undoubtedly pave the way for innovative discoveries and advancements in the realm of biochemistry.
FAQs
1. What is competitive inhibition?
Competitive inhibition is a type of enzyme inhibition where a molecule, known as a competitive inhibitor, competes with the substrate for binding to the active site of an enzyme. This competition reduces the rate of the enzyme-catalyzed reaction.
2. How does competitive inhibition affect enzyme kinetics?
Competitive inhibition increases the apparent KM (Michaelis constant) of the enzyme, meaning that higher substrate concentrations are needed for the enzyme to reach half of its maximal velocity. However, it does not affect the maximum velocity (Vmax) of the reaction.
3. Can competitive inhibition be reversible?
Yes, competitive inhibition is reversible. The competitive inhibitor can dissociate from the enzyme, allowing the substrate to bind and resume the enzymatic reaction. This reversibility makes competitive inhibition a potentially useful target for drug development.
4. Are there any real-world applications of competitive inhibition?
Absolutely! Competitive inhibitors have been utilized in pharmaceutical research to develop drugs that selectively target specific enzymes. By designing competitive inhibitors that bind to disease-associated enzymes, it is possible to regulate their activity and potentially treat various diseases.
5. Are there any examples of competitive inhibition in everyday life?
Yes, many common drugs act as competitive inhibitors. For example, statins used to lower cholesterol levels work by competitively inhibiting the enzyme HMG-CoA reductase, which plays a crucial role in cholesterol synthesis.
Competitive inhibition is just one fascinating aspect of enzyme kinetics. Noncompetitive inhibition, another intriguing phenomenon, affects enzymes differently by binding to sites other than the active site. Enzyme inhibition, in general, plays a crucial role in regulating biochemical processes and has far-reaching implications in various fields. Exploring these topics further will provide a deeper understanding of the complex world of enzymes and their interactions with inhibitors.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
