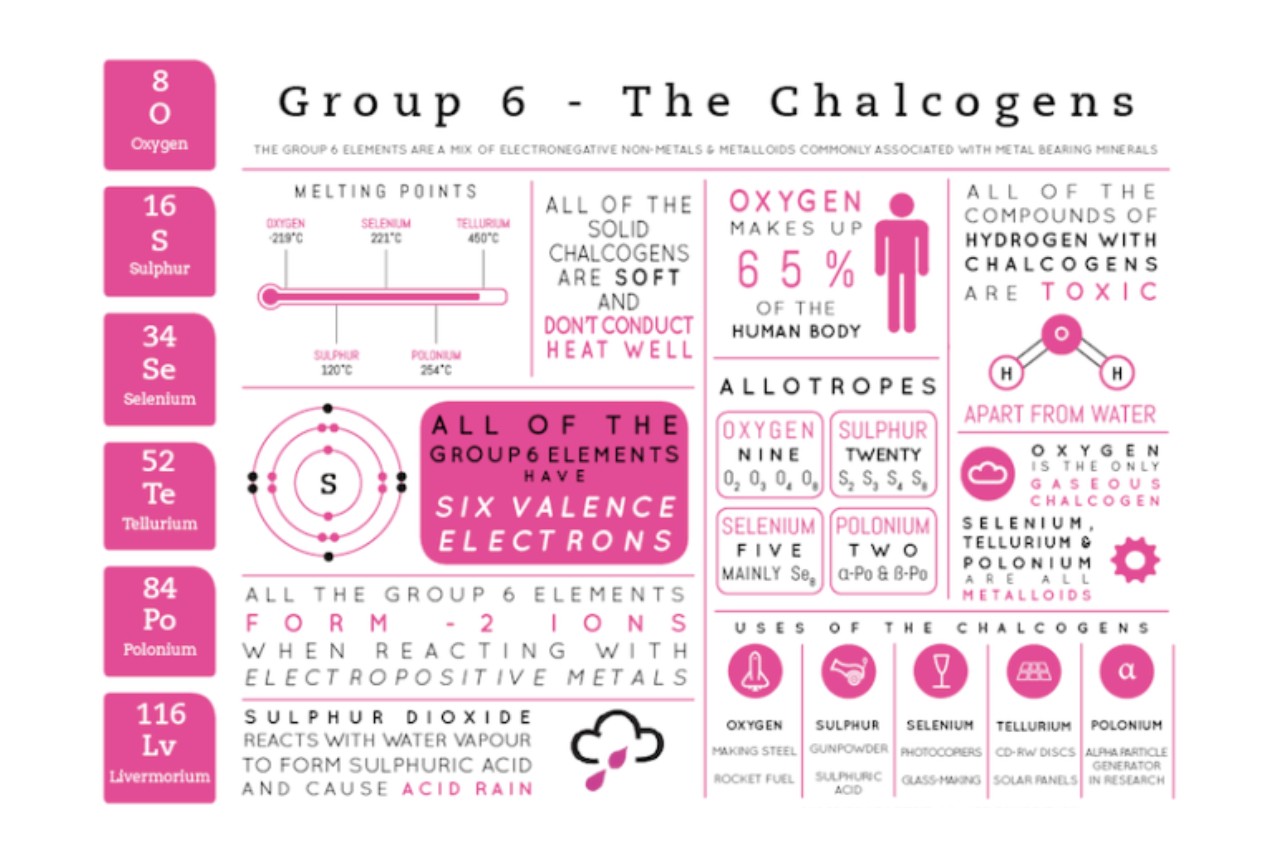
Chalcogens, also known as the oxygen family, are a group of elements in the periodic table that share similar properties and characteristics. They include oxygen, sulfur, selenium, tellurium, and polonium. These elements play a crucial role in various aspects of our lives, from the air we breathe to the technology we use.
In this article, we will explore 16 fascinating facts about chalcogens that will not only enhance your understanding of these elements but also showcase their importance in everyday life. From their discovery to their unique chemical behaviors, we will delve into the world of chalcogens and uncover interesting tidbits that make them truly captivating.
Key Takeaways:
- Chalcogens, like oxygen and sulfur, are essential for life and have diverse industrial and environmental applications, making them fascinating elements with unique properties and historical significance.
- Chalcogens, with their distinct smells, semiconductor properties, and medicinal effects, have played a crucial role in scientific discoveries and technological advancements, shaping our understanding of chemistry and the world around us.
Chalcogens belong to group 16 of the periodic table.
Chalcogens are a group of elements consisting of oxygen, sulfur, selenium, tellurium, and polonium. They are located in group 16, also known as group VIA, of the periodic table.
The name “chalcogen” comes from the Greek word “chalcos”.
The term “chalcogen” is derived from the Greek word “chalcos”, which means “ore” or “copper.” This name was given to the elements in this group due to their association with metal ores.
Oxygen is the most abundant chalcogen on Earth.
Oxygen, with the chemical symbol O, is the most abundant element in the chalcogen group. It makes up approximately 46% of the Earth’s crust by mass.
Chalcogens have diverse chemical properties.
Chalcogens exhibit a wide range of chemical properties. For example, oxygen is a highly reactive nonmetal, while sulfur is a yellow, brittle solid. They can form various compounds and participate in different chemical reactions.
Chalcogens play vital roles in biological systems.
Chalcogens are essential for life as they are involved in many biological processes. For instance, oxygen is crucial for respiration, while selenium is a necessary component of certain enzymes.
Sulfur has a distinct smell.
Sulfur is famously known for its strong and unpleasant odor, resembling that of rotten eggs. This characteristic smell is often associated with volcanic activity and certain industrial processes.
Tellurium is a semiconductor.
Tellurium, one of the rarer chalcogens, possesses semiconductor properties. It is used in various electronic devices, such as solar cells, infrared detectors, and rewritable optical discs.
Chalcogens have multiple oxidation states.
Chalcogens can exhibit different oxidation states, ranging from -2 to +This versatility allows them to form a wide variety of compounds with other elements, contributing to their importance in chemical reactions.
Polonium is highly radioactive.
Polonium, the heaviest and least stable element in the chalcogen group, is highly radioactive. Its radioactive properties make it useful in various applications, such as in static eliminators and as a heat source in space probes.
Chalcogens have diverse industrial applications.
Chalcogens find applications in numerous industries. For example, sulfur is used in the production of fertilizers and rubber, while selenium is utilized in photocopiers and solar cells.
Chalcogens can form compounds with metals.
Chalcogens have the ability to form compounds with metals, known as metal chalcogenides. These compounds often possess interesting electrical and magnetic properties and are employed in various technological advancements.
Chalcogens can combine to form minerals.
Chalcogens can combine with other elements to form minerals. For example, the combination of oxygen and silicon leads to the formation of silicates, which are the most abundant minerals in the Earth’s crust.
Chalcogens are important for environmental processes.
Chalcogens play crucial roles in environmental processes, such as the sulfur cycle. They are involved in the decomposition of organic matter, the formation of sulfates, and the production of greenhouse gases.
Chalcogen compounds have medicinal properties.
Several chalcogen compounds have medicinal properties and are used in pharmaceuticals. For example, selenium compounds are known for their antioxidant and anticancer effects.
Chalcogens have interesting allotropes.
Chalcogens can exist in different allotropes, which are forms of the same element with different molecular structures. For instance, oxygen exists as O2 (oxygen gas) and O3 (ozone), each with distinct properties.
Chalcogens have fascinating historical significance.
The study and use of chalcogens have played a significant role in the history of science and technology. From the discovery of oxygen by Joseph Priestley to the development of selenium photovoltaic cells, chalcogens have continuously pushed the boundaries of knowledge and innovation.
Conclusion
Chalcogens, the elements in group 16 of the periodic table, are truly fascinating. From their unique properties and applications to their importance in various industries, these elements play a vital role in our everyday lives.
Through this article, we have explored 16 intriguing facts about chalcogens. We have learned about their diverse range of compounds, their presence in essential substances like water and proteins, and their contributions to the environment and technology.
Chalcogens, from oxygen to polonium, offer a glimpse into the wondrous world of chemistry. Their ability to form stable compounds and exhibit multiple oxidation states makes them indispensable in chemical reactions and reactions.
As we delve deeper into the realm of chemistry, let us continue to appreciate the fascinating elements that shape our world and propel scientific advancements forward.
FAQs
1. What are chalcogens?
Chalcogens are the elements located in group 16 of the periodic table. They include oxygen, sulfur, selenium, tellurium, and polonium.
2. What are the properties of chalcogens?
Chalcogens exhibit a diverse range of properties. They can exist in different oxidation states, form stable compounds, and display both non-metallic and metallic characteristics.
3. What are some applications of chalcogens?
Chalcogens have various applications in different industries. For example, oxygen is essential for respiration and combustion, sulfur is used in the production of fertilizers and dyes, and selenium is employed in the manufacture of solar cells and electronic devices.
4. Are chalcogens found in nature?
Yes, chalcogens are found abundantly in nature. Oxygen is the most abundant element on Earth, while sulfur is present in minerals and petroleum. Selenium and tellurium are often extracted as byproducts of copper refining, and polonium is a rare radioactive element.
5. Are chalcogens toxic?
Some chalcogens, like oxygen and sulfur, are essential for life and are not toxic in normal amounts. However, certain compounds of selenium, tellurium, and polonium can be toxic in large quantities.
6. How are chalcogens used in the environment?
Chalcogens contribute to various environmental processes. For example, oxygen is crucial for the respiration of living organisms and the formation of the ozone layer. Sulfur compounds play a role in the sulfur cycle, and selenium helps in detoxifying heavy metals in the environment.
7. Can chalcogens form compounds with other elements?
Yes, chalcogens have the ability to form compounds with many other elements. For instance, oxygen forms oxides, sulfur forms sulfides, and tellurium forms tellurides.
8. Can chalcogens conduct electricity?
Chalcogens can exhibit both metallic and non-metallic characteristics. While some chalcogens like sulfur and selenium are poor conductors of electricity, others like tellurium and polonium can behave as semiconductors or even metallic conductors.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
