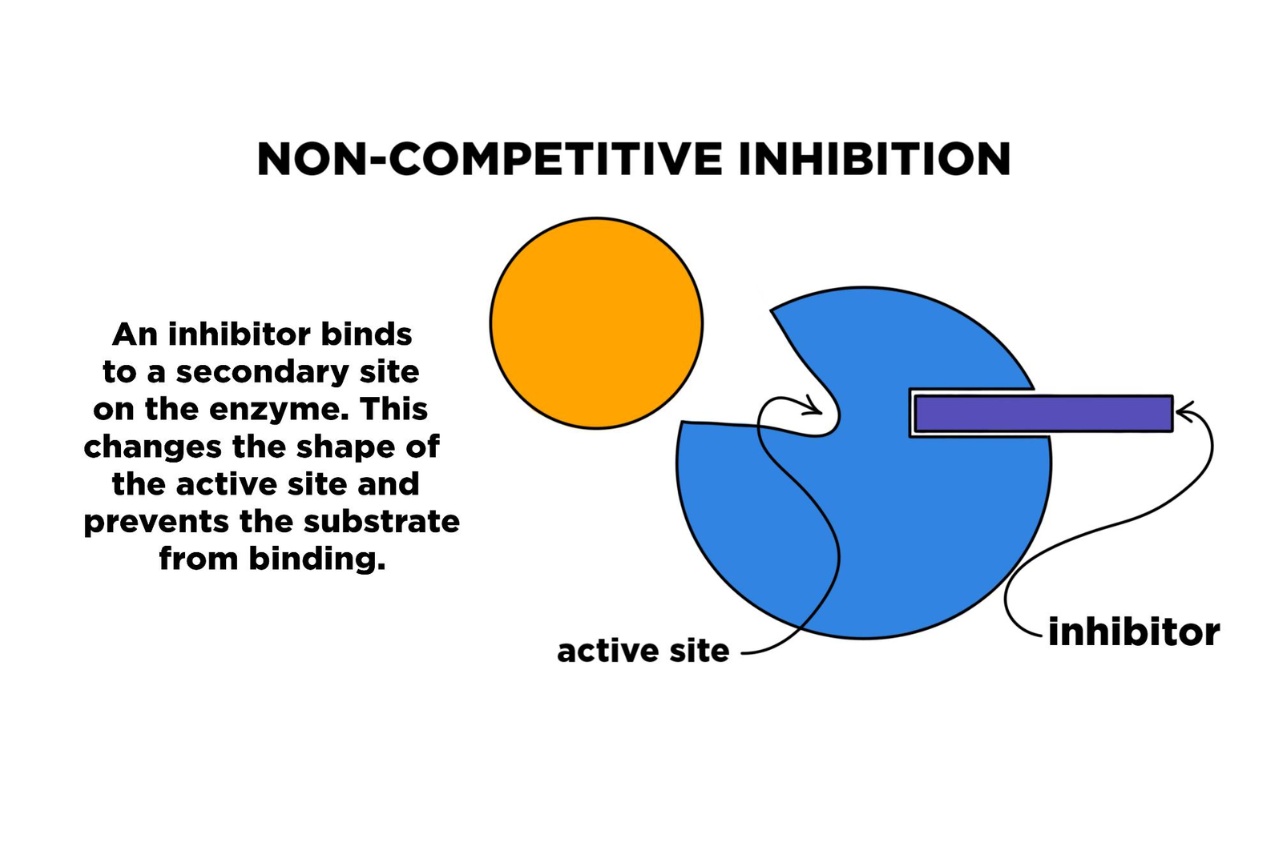
Non-competitive inhibition is a fascinating concept in the field of chemistry that often leaves scientists puzzled and intrigued. It is a unique form of enzyme inhibition that deviates from the more commonly known competitive inhibition. In this intriguing phenomenon, the inhibitor molecule binds to an allosteric site on the enzyme, rather than the active site, causing a change in the enzyme’s structure and ultimately interfering with its function.
In this article, we will delve into the enigmatic world of non-competitive inhibition and explore eight intriguing facts about this phenomenon. From its mechanism of action to its impact on enzyme kinetics, we will unravel the mysteries surrounding non-competitive inhibition and discover its significance in various biochemical processes.
Key Takeaways:
- Non-competitive inhibition is like a secret agent sneaking up on enzymes and changing their shape, making them less effective without directly competing with the substrate. It’s a mysterious and unexpected regulatory mechanism in biology!
- Scientists are like detectives trying to unravel the mysteries of non-competitive inhibition. They use advanced tools to understand how enzymes and inhibitors interact, hoping to uncover new ways to treat diseases and unlock the secrets of biochemistry.
The Mysterious Mechanism
Non-competitive inhibition is a fascinating phenomenon that occurs when an inhibitor molecule binds to the enzyme at a site other than the active site. This binding causes a change in the shape of the enzyme, rendering it less effective in catalyzing the reaction, but without directly competing with the substrate. How exactly this inhibition occurs remains a mystery to scientists.
Unexpected Effects
Non-competitive inhibitors can have unexpected effects on enzyme activity. Unlike competitive inhibition, where increasing substrate concentration can overcome the inhibitory effect, non-competitive inhibition cannot be overcome by increasing substrate concentration. This makes non-competitive inhibition a unique and enigmatic regulatory mechanism.
Varied Targets
Non-competitive inhibition can target a wide range of enzymes and biological processes. It plays a crucial role in the regulation of metabolic pathways, signal transduction, and even drug interactions. Understanding this complex mechanism is essential for developing effective treatments for various diseases.
Allosteric Regulation
Non-competitive inhibition is often associated with allosteric regulation, where the inhibitor molecule binds to a specific allosteric site on the enzyme. This binding induces a conformational change in the enzyme, altering its activity. This unique mode of regulation adds another layer of complexity to the understanding of non-competitive inhibition.
Irreversible Inhibition
In some cases, non-competitive inhibition can be irreversible, meaning the inhibitor molecule binds permanently to the enzyme, rendering it permanently inactive. This irreversible inhibition can have profound effects on cellular processes and can be used as a strategy in drug development.
Potential Therapeutic Applications
Non-competitive inhibitors have shown promise as potential therapeutics in treating various diseases, including cancer. By specifically targeting enzymes involved in abnormal cell growth or signaling pathways, non-competitive inhibitors offer a unique approach to regulating these processes and potentially inhibiting disease progression.
Unraveling the Mysteries
Scientists are constantly researching non-competitive inhibition to unravel its mysteries and understand its mechanisms in more detail. Through advanced techniques such as structural biology and computational modeling, researchers aim to shed light on the complex interactions between enzymes and inhibitors, leading to new breakthroughs in drug design and medical treatments.
A Never-Ending Puzzle
Despite significant progress in understanding non-competitive inhibition, many questions still remain unanswered. The intricacies of this regulatory mechanism continue to challenge scientists, inspiring further research and exploration into the enigmatic world of non-competitive inhibition.
Conclusion
Non-competitive inhibition is an intriguing phenomenon in the field of chemistry. Its unique mechanism of action and effects on enzyme activity make it an important concept to understand. While we have explored eight enigmatic facts about non-competitive inhibition in this article, it is just the tip of the iceberg. There is still much more to be discovered and explored in this fascinating area of study.
FAQs
Q: What is non-competitive inhibition?
A: Non-competitive inhibition is a type of enzyme inhibition where the inhibitor binds to a site on the enzyme that is different from the active site. This binding alters the enzyme’s structure and prevents it from carrying out its normal function.
Q: How does non-competitive inhibition differ from competitive inhibition?
A: In competitive inhibition, the inhibitor competes with the substrate for binding at the active site of the enzyme, while in non-competitive inhibition, the inhibitor binds to a different site on the enzyme, causing structural changes that impede enzyme activity.
Q: What are some examples of non-competitive inhibitors?
A: Some examples of non-competitive inhibitors include heavy metals like mercury and lead, as well as certain drugs and toxins that can bind to enzymes and inhibit their activity.
Q: How does non-competitive inhibition affect enzyme kinetics?
A: Non-competitive inhibition decreases the Vmax (maximum reaction rate) of the enzyme without affecting the Km (substrate concentration at half-maximal velocity). This indicates that non-competitive inhibition does not change the affinity of the enzyme for its substrate.
Q: Can non-competitive inhibition be reversed?
A: Unlike competitive inhibition, non-competitive inhibition is typically irreversible. The inhibitor binds to the enzyme in a manner that cannot be easily displaced, rendering the enzyme permanently inactive.
Q: What are the physiological roles of non-competitive inhibition?
A: Non-competitive inhibition plays an essential role in regulating enzyme activity and controlling metabolic pathways in cells. It helps maintain homeostasis and ensures that biochemical processes occur at appropriate rates.
Q: How can non-competitive inhibition be overcome?
A: Since non-competitive inhibition is usually irreversible, it cannot be easily overcome. However, increasing the concentration of the substrate may reduce the impact of the inhibitor by increasing the chance of substrate binding to active enzyme sites.
Q: Is non-competitive inhibition beneficial or harmful?
A: Non-competitive inhibition can have both positive and negative effects. In certain situations, it serves as a regulatory mechanism, allowing cells to fine-tune enzyme activity. However, non-competitive inhibitors can also interfere with essential cellular processes, leading to detrimental effects.
Non-competitive inhibition remains an enigma, leaving scientists puzzled and eager to explore further. While this article shed light on some captivating aspects, the world of enzyme inhibition holds even more surprises waiting to be uncovered. For those intrigued by the complexities of biochemical reactions, our next article delves into the fascinating realm of enzyme inhibition, revealing 14 facts that will leave you in awe. So, if you're ready to expand your knowledge and be amazed by the intricacies of life at the molecular level, keep reading and prepare to be captivated by the wonders of enzyme inhibition.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
