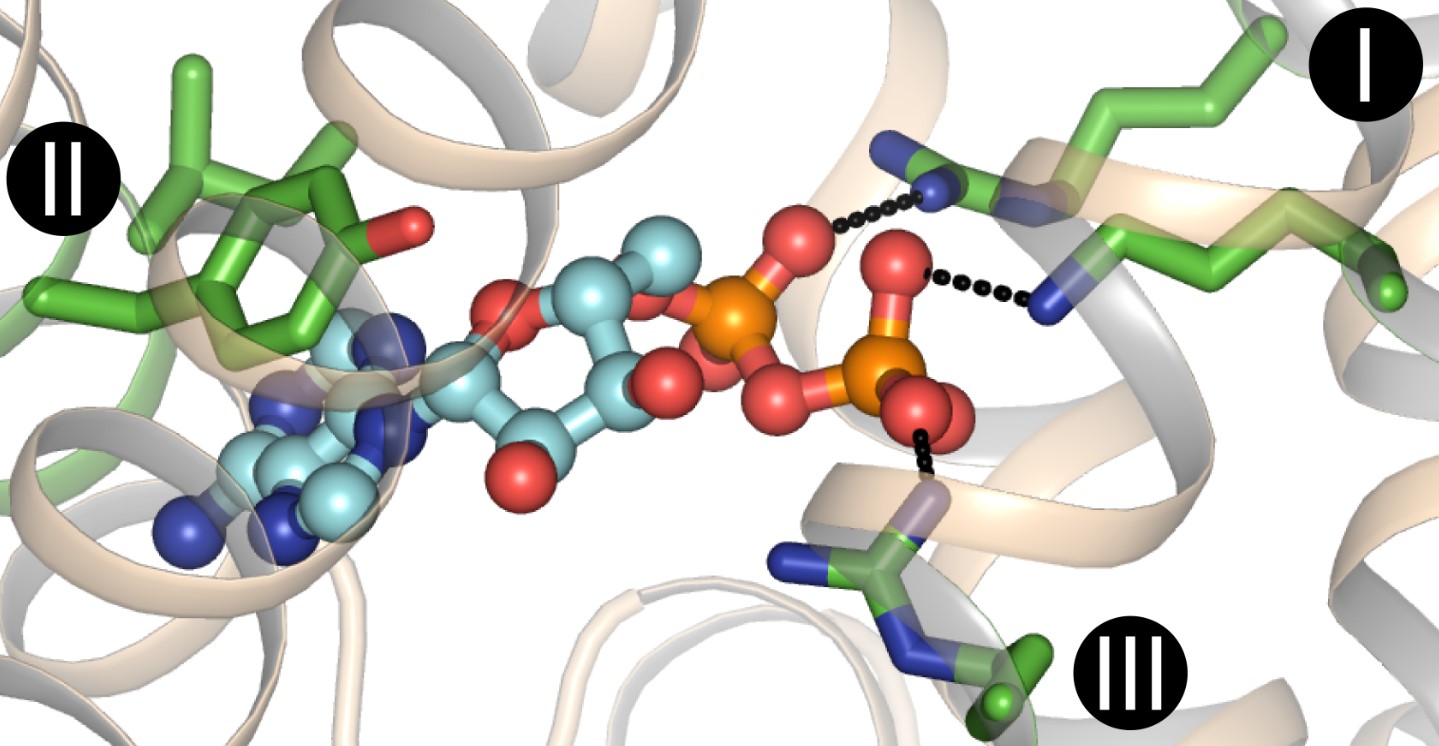
Substrate binding is a fundamental process in biology that plays a crucial role in various biological systems. It refers to the specific interaction between a substrate molecule and its corresponding binding site on a protein or enzyme. Substrate binding is essential for the successful execution of many biological functions, such as enzymatic catalysis, signal transduction, and transport across cell membranes.
While substrate binding may seem like a straightforward concept, there are numerous surprising aspects and intricacies associated with this phenomenon. In this article, we will explore nine intriguing and lesser-known facts about substrate binding that will expand your understanding of this fundamental biological process. From allosteric regulation to induced fit mechanisms, these facts highlight the complexity and diversity of substrate binding and its significance in cellular processes.
Key Takeaways:
- Substrate binding is like a lock and key, where enzymes and specific substrates fit together perfectly to kickstart important chemical reactions in living organisms.
- Understanding substrate binding can help scientists develop new medicines that target this process, potentially leading to innovative treatments for various diseases.
Substrate binding is a crucial step in enzymatic reactions.
Enzymes, the molecular machines responsible for catalyzing biochemical reactions, rely on substrate binding to initiate the conversion of substrates into products. The active site of an enzyme interacts with the substrate, creating an enzyme-substrate complex, which allows for the precise positioning and orientation necessary for the reaction to occur.
Substrate binding can be highly specific.
Many enzymes exhibit high substrate specificity, only recognizing and binding to particular substrates that fit into their active sites like a lock and key. This specificity ensures that reactions occur selectively, preventing wasteful or detrimental interactions.
Substrate binding can induce conformational changes.
The binding of a substrate to an enzyme can trigger structural rearrangements within the enzyme’s active site. These conformational changes often optimize the enzyme-substrate interaction, leading to increased catalytic efficiency and enhancing the overall reaction rate.
Substrate binding can be reversible.
In some cases, substrate binding is reversible, meaning that the substrate can bind and dissociate from the enzyme multiple times. This reversible binding enables fine-tuning of enzymatic activity, allowing for dynamic regulation of cellular processes.
Substrate binding can be influenced by cofactors.
Certain enzymes require the presence of additional non-protein molecules called cofactors to facilitate substrate binding. These cofactors can be metal ions or organic compounds that contribute to the enzyme’s catalytic capabilities and assist in the proper alignment and stabilization of the substrate.
Substrate binding can involve multiple binding sites.
In some cases, enzymes possess multiple binding sites where substrates can bind simultaneously or sequentially. This enables complex biological reactions that involve multiple substrates or the transfer of functional groups between substrates.
Substrate binding can be regulated by allosteric interactions.
Allosteric regulation occurs when the binding of a molecule to one site on an enzyme affects the affinity or activity of another site. In substrate binding, allosteric interactions can modulate the enzyme’s ability to bind and interact with its substrate, thereby influencing the rate of the enzymatic reaction.
Substrate binding can be influenced by pH and temperature.
The physicochemical conditions such as pH and temperature can significantly impact the binding affinity and enzymatic activity. Optimal pH and temperature conditions ensure the proper functioning of enzymes and allow for efficient substrate binding and reaction kinetics.
Substrate binding can be a target for drug development.
Given the vital role of substrate binding in enzymatic reactions, it has become an attractive target for drug development. By designing molecules that selectively bind to specific enzyme substrates or disrupt the binding process, researchers can develop innovative therapies to modulate biological pathways and treat various diseases.
Conclusion
In conclusion, substrate binding is a fascinating and essential process in biology. It involves the interaction between a substrate molecule and its corresponding binding site on a protein or enzyme. While the concept of substrate binding may seem straightforward, there are several surprising facts that highlight its complexity.
From allosteric effects to binding affinity, substrate binding plays a crucial role in various cellular processes, including enzymatic reactions, signal transduction, and drug interactions. Understanding the intricacies of substrate binding can provide valuable insights into disease mechanisms and aid in the development of targeted therapies.
By delving deeper into the world of substrate binding, researchers can uncover new breakthroughs and advancements in the field of biology. It is an area of study that continues to captivate scientists and offers exciting prospects for further research and discovery.
FAQs
Q: What is substrate binding?
A: Substrate binding refers to the interaction between a substrate molecule and its corresponding binding site on a protein or enzyme.
Q: Why is substrate binding important?
A: Substrate binding is essential for various biological processes, including enzymatic reactions, signal transduction, and drug interactions.
Q: What factors influence substrate binding?
A: Factors such as binding affinity, allosteric effects, and structural compatibility between the substrate and binding site can influence substrate binding.
Q: How does substrate binding affect enzyme activity?
A: Substrate binding is a crucial step in enzymatic reactions, as it allows for the formation of an enzyme-substrate complex and facilitates the conversion of substrates into products.
Q: Can substrate binding be regulated?
A: Yes, substrate binding can be regulated through various mechanisms, including allosteric regulation, covalent modifications, and the presence of effector molecules.
Q: Are there any specific diseases or conditions associated with abnormalities in substrate binding?
A: Yes, abnormalities in substrate binding can contribute to various diseases, including genetic disorders and metabolic diseases.
Q: How is substrate binding studied?
A: Substrate binding is studied using various techniques, such as binding assays, X-ray crystallography, molecular modeling, and biochemical experiments.
Q: Can substrate binding be targeted for therapeutic interventions?
A: Yes, targeting substrate binding sites on proteins or enzymes can be a potential strategy for developing therapeutic interventions and designing drugs.
Q: What are some recent advancements in the understanding of substrate binding?
A: Recent advancements include the discovery of novel binding sites, the development of computational tools for predicting binding interactions, and the exploration of allosteric modulation in substrate binding.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
