The Michaelis-Menten kinetics is a fundamental concept in enzymology and plays a crucial role in understanding the mechanisms of enzyme-catalyzed reactions. Named after the biochemists Leonor Michaelis and Maud Menten, this mathematical model describes the relationship between an enzyme’s substrate concentration, reaction rate, and the enzyme-substrate complex formation.
While this model may seem straightforward, there are several surprising facts about Michaelis-Menten kinetics that researchers have uncovered over the years. In this article, we will delve into 15 fascinating facts that shed light on the intricacies of this kinetic model. From understanding the significance of the Michaelis constant (Km) to exploring the effects of enzyme inhibitors on reaction rates, these facts will deepen your knowledge of this important area of chemistry.
Key Takeaways:
- The Michaelis-Menten equation, created in 1913, revolutionized our understanding of how enzymes work, helping scientists study and predict enzyme behavior for over a century.
- This equation, based on simple chemical principles, has practical applications in drug discovery and remains a vital tool for biochemists, paving the way for enzyme simulations and the development of new therapies.
The Michaelis-Menten equation was first proposed in 1913.
In 1913, biochemist Leonor Michaelis and physician Maud Menten introduced the Michaelis-Menten equation, a mathematical model that relates the rate of an enzyme-catalyzed reaction to the concentration of the substrate.
It revolutionized the understanding of enzyme kinetics.
The Michaelis-Menten equation provided a breakthrough in enzyme kinetics, allowing scientists to better comprehend the mechanism and behavior of enzymes. It laid the foundation for further research in the field.
The equation assumes a simple enzyme-substrate interaction.
The Michaelis-Menten equation assumes that the enzyme-substrate interaction follows a simple reversible reaction, where the enzyme binds to the substrate to form an enzyme-substrate complex, which then converts into the desired product.
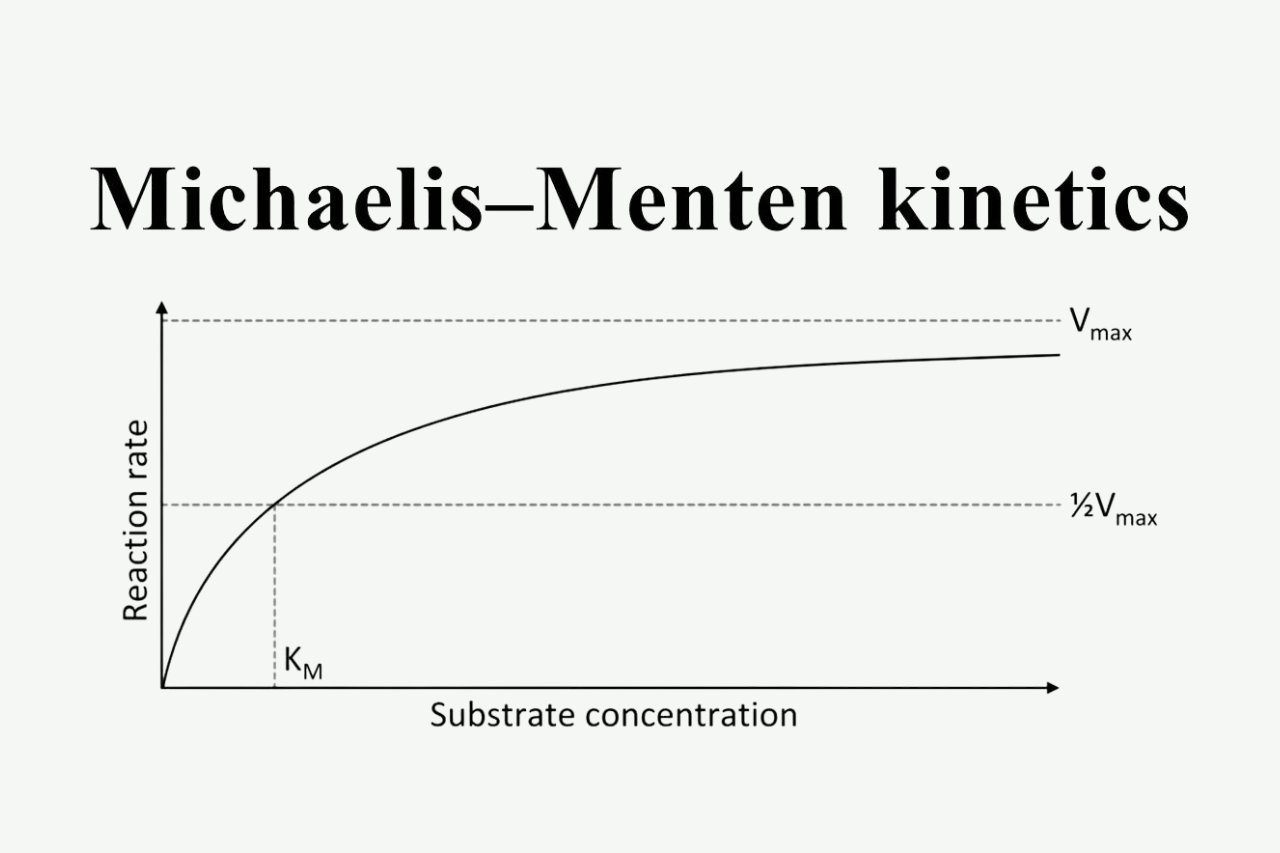
It introduced the concepts of KM and Vmax.
The Michaelis-Menten equation introduced two essential concepts: KM, the Michaelis constant, which represents the substrate concentration at which the reaction rate is half of its maximum, and Vmax, the maximum reaction rate achievable at saturating substrate concentrations.
The equation holds true for many enzyme-catalyzed reactions.
Despite its simplicity, the Michaelis-Menten equation is widely applicable and accurately describes the kinetics of numerous enzyme-catalyzed reactions, ranging from metabolic pathways to drug metabolism.
It assumes steady-state conditions.
The Michaelis-Menten kinetics assumes that the concentrations of the enzyme and the substrate remain constant during the course of the reaction, known as steady-state conditions. This simplification allows for easier mathematical analysis.
The equation can be modified for more complex enzyme mechanisms.
While the Michaelis-Menten equation provides a useful approximation for many enzymes, it can be modified to accommodate more intricate enzyme mechanisms, such as cooperative substrate binding or substrate inhibition.
The equation is derived from basic principles of chemical kinetics.
The derivation of the Michaelis-Menten equation is based on fundamental principles of chemical kinetics, such as the law of mass action and the assumption of rapid equilibrium between the enzyme, substrate, and enzyme-substrate complex.
It helps determine enzyme efficiency.
The Michaelis-Menten equation allows researchers to determine important parameters such as enzyme efficiency, which is quantified by the catalytic efficiency (kcat/KM) of an enzyme, representing the rate of product formation per unit enzyme-substrate complex.
The equation has limitations for enzymes with multiple substrates.
The Michaelis-Menten equation is primarily designed for single-substrate reactions. Enzymes with multiple substrates often require more complex kinetic models to accurately describe their behavior.
It inspired the development of alternative kinetic models.
While the Michaelis-Menten equation is widely used, it has inspired the development of various alternative kinetic models, such as the Briggs-Haldane model and the Lineweaver-Burk plot, which offer enhanced accuracy for specific enzyme systems.
The equation assumes no product inhibition.
In its original form, the Michaelis-Menten equation does not account for product inhibition, a phenomenon where the product of a reaction inhibits the enzyme activity. Modified versions of the equation exist to incorporate product inhibition if required.
It has practical applications in drug discovery.
Understanding the kinetics of enzyme interactions is vital in drug discovery, as it helps scientists identify potential inhibitors or activators for specific enzymes. The Michaelis-Menten equation contributes to this process.
It paved the way for enzyme kinetics simulations.
With the aid of computer simulations, the Michaelis-Menten equation has been crucial in modeling and predicting enzyme behavior. These simulations offer valuable insights into enzyme kinetics and aid in the design of optimized enzymes for various applications.
It remains an indispensable tool in biochemistry.
Even after more than a century since its inception, the Michaelis-Menten equation continues to be an indispensable tool in biochemistry, providing a solid foundation for the study of enzymatic reactions and the development of novel therapeutics.
Conclusion
In conclusion, Michaelis-Menten kinetics is a fascinating and essential concept in the field of enzymology. It provides insights into the speed and efficiency of enzyme-substrate interactions, helping us understand the underlying mechanisms of enzymatic reactions. Throughout this article, we have explored 15 surprising facts about Michaelis-Menten kinetics, shedding light on its significance and applications.From understanding the concept of enzyme velocity to the derivation of the Michaelis-Menten equation, we have dived into the intricate details of this kinetic model. We have discovered how the substrate concentration affects the rate of enzymatic reactions and the various parameters involved, such as the maximum reaction rate (Vmax) and the Michaelis constant (Km).Furthermore, we have explored the limitations of the Michaelis-Menten model and how it can be modified to account for various scenarios, such as enzyme inhibition and allosteric regulation. These insights are crucial for researchers and scientists in designing experiments, predicting enzyme behavior, and developing potential therapeutic interventions.Overall, Michaelis-Menten kinetics is a powerful tool that continues to shape our understanding of enzymatic reactions and catalysis. By delving into its intricacies, we uncover the fascinating world of enzymology, paving the way for advancements in various scientific disciplines.
FAQs
1. What is Michaelis-Menten kinetics?
Michaelis-Menten kinetics is a mathematical model used to describe the rate of enzymatic reactions. It provides insights into the relationship between the substrate concentration and the rate of reaction.
2. Who developed the Michaelis-Menten model?
The Michaelis-Menten model was developed by Leonor Michaelis and Maud Menten in 1913. They proposed this model to explain the enzymatic reaction kinetics.
3. What is the Michaelis constant (Km)?
The Michaelis constant (Km) is a measure of the affinity between an enzyme and its substrate. It represents the substrate concentration at which the reaction rate is half of the maximum reaction rate (Vmax).
4. Can the Michaelis-Menten model account for inhibitory effects?
No, the original Michaelis-Menten model does not account for inhibitory effects. However, modifications can be made to the model to include inhibition, such as competitive, non-competitive, and uncompetitive inhibition.
5. How is the Vmax determined in Michaelis-Menten kinetics?
The Vmax (maximum rate of reaction) can be determined by obtaining the reaction rate at saturating substrate concentrations, where the enzyme is working at its maximum capacity.
6. What are the assumptions made in the Michaelis-Menten model?
The Michaelis-Menten model assumes that the enzyme-substrate complex is formed reversibly, the concentration of the enzyme-substrate complex remains constant throughout the reaction, and the enzymatic reaction occurs in a single step.
7. Is the Michaelis-Menten model applicable to all enzymes?
The Michaelis-Menten model is applicable to enzymes that follow simple saturation kinetics. However, for enzymes that exhibit complex behavior, alternative kinetic models may be required to describe their reactions accurately.
Michaelis-Menten kinetics is just the beginning of your journey into the fascinating world of biochemistry. Dive deeper into the factors influencing reaction rates and uncover more surprising facts. Explore the intricate mechanisms of enzyme regulation and how they shape cellular processes. Biochemistry holds a treasure trove of extraordinary facts waiting to be discovered, so keep reading and expand your knowledge in this captivating field.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


