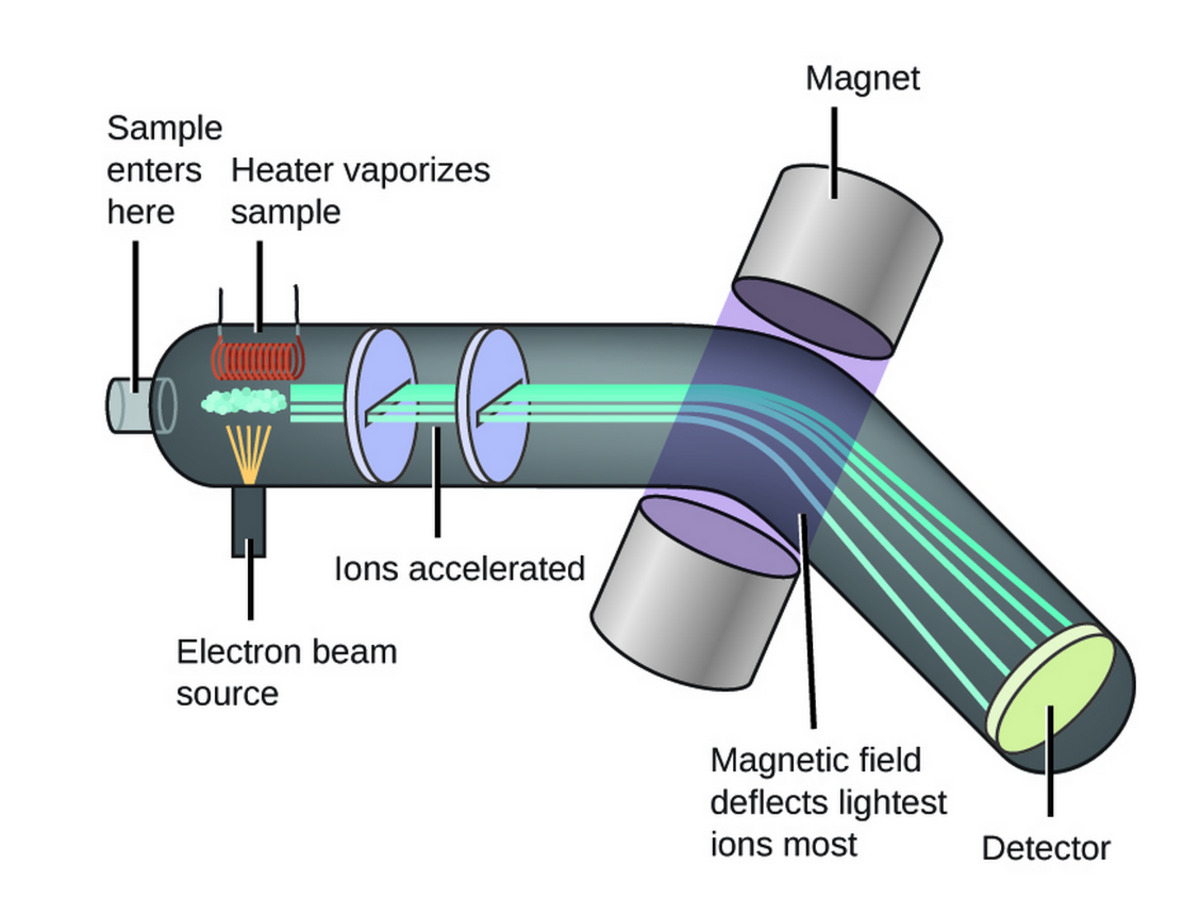
Mass spectrometry (MS) is a powerful analytical technique that has revolutionized the field of chemistry. It is a fascinating method used to study the composition, structure, and properties of molecules. With its ability to provide precise and detailed information about the mass-to-charge ratio of ions, MS has become an indispensable tool for researchers in various industries.
In this article, we will explore 19 unbelievable facts about mass spectrometry. From its origins to its wide-ranging applications, this advanced technology has continuously evolved and pushed the boundaries of scientific discovery. Whether you’re a chemistry enthusiast or simply curious about the wonders of modern science, these facts will astound you and deepen your appreciation for the incredible world of mass spectrometry.
Key Takeaways:
- Mass spectrometry is a powerful tool that can determine the molecular weight, identify unknown compounds, and provide structural information, revolutionizing fields like drug development and environmental science.
- With its ability to analyze complex mixtures, detect trace amounts of substances, and study protein structures, mass spectrometry continues to evolve and make a significant impact in various scientific and real-world applications.
Mass spectrometry is a powerful analytical technique.
Mass spectrometry (MS) is a versatile technique used in chemistry and biochemistry to analyze the chemical and physical properties of molecules. It is widely used in various fields such as pharmaceuticals, forensics, environmental science, and proteomics.
MS can determine the molecular weight of a compound.
One of the key applications of mass spectrometry is determining the molecular weight of a compound. By ionizing a sample and measuring the mass-to-charge ratio of the resulting ions, MS can provide precise information about the mass of a molecule.
MS can identify unknown compounds.
Mass spectrometry is a powerful tool for identifying unknown compounds. By comparing the mass spectra of the sample to a database of known compounds, scientists can determine the identity of the substances present in a sample.
MS can provide structural information.
Mass spectrometry can also provide information about the structure of a molecule. By fragmenting the ions and analyzing their mass to charge ratios, scientists can gain insight into the connectivity and arrangement of atoms within a molecule.
MS can quantify the amount of a compound in a sample.
Mass spectrometry can be used to measure the amount of a particular compound present in a sample. This quantitative analysis is essential in fields such as clinical diagnostics, drug development, and environmental monitoring.
MS can analyze complex mixtures.
Mass spectrometry excels at analyzing complex mixtures of compounds. By separating the components of a mixture and analyzing their mass spectra, scientists can gain a comprehensive understanding of the composition and concentration of the mixture.
MS can detect trace amounts of substances.
Mass spectrometry is highly sensitive and can detect trace amounts of substances. This makes it invaluable in applications such as detecting drugs in forensic samples, identifying pollutants in environmental samples, and monitoring contaminants in food and beverages.
MS can be coupled with other analytical techniques.
Mass spectrometry can be coupled with other analytical techniques, such as gas chromatography (GC-MS) or liquid chromatography (LC-MS), to enhance its capabilities. This allows for more comprehensive analysis and greater sensitivity.
MS can be used to study protein structures.
Mass spectrometry plays a crucial role in proteomics, the study of proteins. It can be used to analyze the structure, modifications, and interactions of proteins, providing valuable insights into their functions and roles in biological processes.
MS is used in drug discovery and development.
Mass spectrometry is widely used in the pharmaceutical industry for drug discovery and development. It helps in the identification of potential drug candidates, characterization of their properties, and evaluation of their stability and metabolism.
MS can be used to study isotopes.
Isotope analysis is another application of mass spectrometry. By measuring the mass differences between isotopes of an element, scientists can determine the origin, age, and isotopic composition of a substance.
MS can identify counterfeit products.
Mass spectrometry is employed in the authentication and quality control of products. By analyzing the molecular composition of a product, MS can detect the presence of counterfeit or substandard materials.
MS has applications in environmental science.
Mass spectrometry is extensively used in environmental science to analyze pollutants, monitor air and water quality, and study the fate and transport of contaminants in the environment.
MS has revolutionized proteomics research.
The development of advanced mass spectrometry techniques has revolutionized proteomics research. It enables the identification and quantification of thousands of proteins, leading to a deeper understanding of complex biological systems.
MS can provide insights into drug metabolism.
Mass spectrometry is crucial in studying drug metabolism, including the breakdown and elimination of drugs from the body. This information helps optimize drug dosages and minimize potential side effects.
MS can analyze small molecules and metabolites.
Mass spectrometry is widely used to analyze small molecules and metabolites in biological samples. This helps in understanding metabolic pathways, disease biomarkers, and the effects of drugs on cellular processes.
MS can be used for elemental analysis.
Mass spectrometry is a powerful technique for elemental analysis. It allows for the identification and quantification of elements and their isotopes, providing valuable information in fields such as geochemistry and material science.
MS can analyze complex carbohydrates.
Mass spectrometry is used to analyze complex carbohydrates, such as glycoproteins and glycolipids. This helps in understanding their structures, functions, and roles in biological processes, including cell-cell interactions and signaling.
MS is constantly evolving.
Mass spectrometry is a dynamic field, continuously evolving with advancements in technology and techniques. Innovations in instrumentation and data analysis continue to expand the capabilities and applications of mass spectrometry.
Overall, the 19 unbelievable facts about mass spectrometry (MS) highlight the versatility, power, and impact of this analytical technique. From analyzing complex mixtures to providing structural insights, from drug discovery to proteomics research, mass spectrometry plays a vital role in advancing scientific knowledge and solving real-world problems.
Conclusion
In conclusion, mass spectrometry (MS) is an amazing scientific technique that has revolutionized the field of chemistry. Its ability to analyze the composition, structure, and properties of various substances has opened new avenues for research and discovery. The 19 unbelievable facts about mass spectrometry highlighted in this article shed light on the immense capabilities and applications of this technique.From its role in identifying unknown substances to its use in forensic investigations and drug development, mass spectrometry has proven to be an invaluable tool in the scientific community. Its ability to provide precise and detailed information about molecules has paved the way for advancements in fields like environmental science, biochemistry, and materials science.As technology continues to advance, we can expect even more remarkable developments in the field of mass spectrometry. Researchers are constantly pushing the boundaries to improve the sensitivity, resolution, and speed of MS instruments, making it an indispensable technique for future scientific breakthroughs.In summary, mass spectrometry has changed the way we analyze and understand the world around us. Its impact on various industries and scientific disciplines is undeniable, and we can only anticipate more exciting discoveries and applications in the years to come.
FAQs
Q: What is mass spectrometry (MS)?
A: Mass spectrometry is a powerful analytical technique used to determine the composition, structure, and chemical properties of substances based on their mass-to-charge ratio.
Q: How does mass spectrometry work?
A: Mass spectrometry involves ionizing a sample, separating the ions based on their mass-to-charge ratio, detecting the ions, and generating a mass spectrum that provides information about the composition and structure of the analyte.
Q: What are the applications of mass spectrometry?
A: Mass spectrometry has a wide range of applications, including drug discovery, environmental analysis, proteomics, metabolomics, forensic investigations, and determining the isotopic composition of elements.
Q: What are the advantages of mass spectrometry?
A: Mass spectrometry offers high sensitivity, selectivity, and accuracy in analyzing complex mixtures. It can provide detailed structural information, quantify trace amounts of substances, and identify unknown compounds.
Q: Is mass spectrometry used in medicine?
A: Yes, mass spectrometry plays a crucial role in medicine, from drug development and pharmacokinetics to disease biomarker discovery and clinical diagnostics.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
