Zero-order reactions are a fascinating topic in chemistry that can leave you in awe of the complexities of chemical kinetics. Understanding the dynamics of reactions is crucial in many fields, including pharmaceuticals, environmental sciences, and industrial processes. Zero-order reactions are unique because the rate of reaction does not depend on the concentration of reactants, making them intriguing and mind-blowing.
In this article, we will delve into the world of zero-order reactions and uncover some mind-blowing facts that will not only expand your knowledge but also ignite your curiosity. From their characteristics to real-life applications, these facts will shed light on the intriguing nature of zero-order reactions and how they play a significant role in numerous chemical processes.
Key Takeaways:
- Zero-order reactions defy the norm by maintaining a constant rate regardless of concentration changes, offering unique insights into complex chemical processes and practical applications in fields like drug delivery.
- Understanding zero-order reactions unlocks a world of possibilities, from designing controlled-release medications to unraveling intricate reaction kinetics, providing valuable insights for scientific discovery and engineering advancements.
The Mysterious Zero-Order Reaction
The zero-order reaction is a fascinating phenomenon in chemistry. Unlike most chemical reactions that are influenced by the concentration of reactants, the rate of a zero-order reaction remains constant regardless of concentration changes.
No Proportional Relationship
One of the mind-blowing aspects of a zero-order reaction is that the rate of reaction is not proportional to the concentration of the reactants. It defies the typical relationship seen in first-order or second-order reactions.
Complex Mechanisms
Zero-order reactions often involve complex mechanisms and multiple steps. This complexity adds to the intrigue and challenges researchers to unravel the underlying processes at play.
Real-life Applications
The concept of zero-order reactions finds its relevance in various real-life applications. It is commonly observed in enzymatic reactions, drug metabolism, and controlled-release formulations.
Concentration Independence
One remarkable aspect of zero-order reactions is their independence from concentration changes. Regardless of the initial concentration, the rate of reaction remains constant over time.
Factors Influencing Zero-Order Reaction
While concentration plays no role in determining the rate of a zero-order reaction, other factors, such as temperature, catalysts, and surface area, can still impact the reaction’s speed.
Half-Life Concept
Although commonly associated with radioactive decay, the concept of half-life can also be applied to zero-order reactions. The half-life of a zero-order reaction is constant, irrespective of the initial concentration.
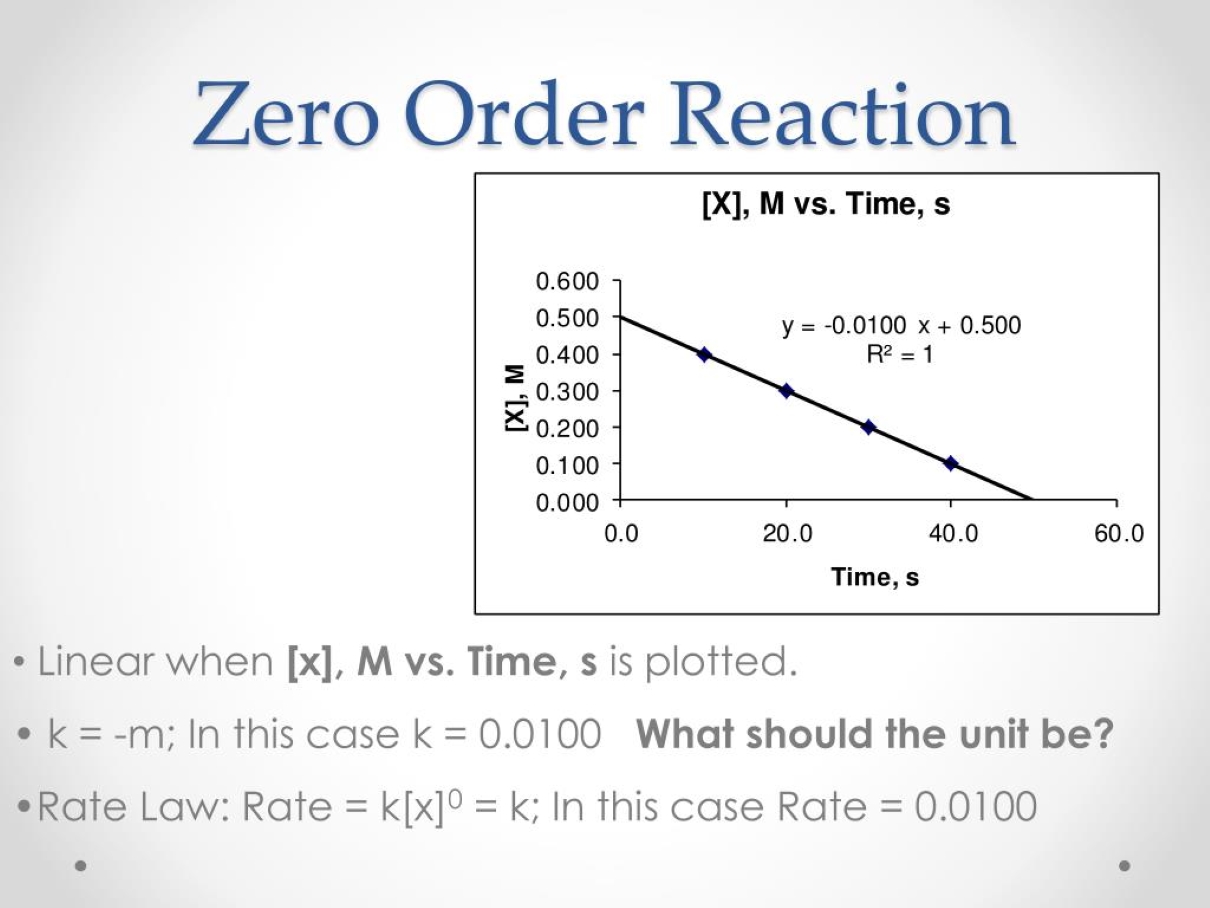
Determining Reaction Order
Identifying a zero-order reaction is an important step in understanding the behavior and kinetics of a chemical reaction. Experimental data, such as the slope of concentration versus time graph, can help determine the order of reaction.
Rate Law Expression
The rate law expression for a zero-order reaction is typically expressed as: Rate = k, where k is the rate constant. This equation emphasizes that the rate of reaction is independent of reactant concentration.
Zero-Order Reactions vs. First-Order Reactions
Zero-order reactions differ significantly from first-order reactions, where the rate is directly proportional to the concentration of a single reactant. Understanding these distinctions helps in predicting and manipulating reaction rates.
Temperature Dependence
Although zero-order reactions are concentration-independent, temperature influences the rate constant (k). Higher temperatures generally lead to increased reaction rates, highlighting the importance of thermodynamics in these reactions.
Engineering Control
The knowledge of zero-order reactions allows engineers to design better processes that require precise control over reaction rates. For example, controlled-release medications utilize zero-order kinetics to ensure optimal drug delivery.
Nonlinear Concentration-Time Relationship
Unlike first-order and second-order reactions with linear concentration-time relationships, zero-order reactions exhibit nonlinear behavior. This peculiarity adds complexity to their analysis and modeling.
Practical Implications
Studying zero-order reactions has practical implications, such as optimizing reaction conditions, designing drug dosage forms, and understanding the lifetimes of certain substances under specific conditions.
Zero-Order Decay
Similar to zero-order reactions, zero-order decay refers to the decay of a substance at a constant rate over time, regardless of the initial quantity. This concept is frequently encountered in radioactive decay processes.
Fundamental Research Tool
Zero-order reactions serve as fundamental tools for researchers investigating reaction kinetics, catalysis, and complex reaction mechanisms. They provide insights into the behavior of chemical systems under unique conditions.
Experimental Strategies
Understanding zero-order reactions requires careful experimental planning. Techniques such as continuous flow reactors, enzyme kinetics analysis, and isothermal conditions facilitate accurate data collection and analysis.
Rate-Determining Steps
In zero-order reactions, the rate-determining step usually involves a complex series of reactions or slow chemical transformations. Identifying these steps is crucial for understanding the overall kinetics of the reaction.
The Road Less Traveled
Zero-order reactions are often considered unconventional and intriguing due to their departure from rate-concentration relationships. Exploring this lesser-known realm of reaction kinetics opens up new avenues for scientific discovery.
These 19 mind-blowing facts about zero-order reactions shed light on the unique properties and behaviors of this fascinating chemical phenomenon. From concentration independence to complex mechanisms, zero-order reactions continue to captivate scientists and offer valuable insights in various fields of study. Whether it’s designing controlled-release medications or unraveling intricate reaction kinetics, understanding the intricacies of zero-order reactions unlocks a world of possibilities.
Conclusion
In conclusion, zero-order reactions are an intriguing aspect of chemistry that involves a reaction rate that remains constant regardless of the concentration of reactants. The concept may seem counterintuitive, but it plays a significant role in various chemical processes.Throughout this article, we have explored some mind-blowing facts about zero-order reactions. We have learned that in a zero-order reaction, the rate of the reaction is independent of the concentration of the reactants, making it a unique phenomenon in chemical kinetics.We have also delved into the implications and applications of zero-order reactions, such as their use in controlled drug release systems and the determination of rate constants.Understanding the intricacies of zero-order reactions expands our knowledge of chemical reactions and their behavior. By grasping the fundamental principles behind these reactions, we can better comprehend the complex world of chemistry.In summary, zero-order reactions challenge our conventional understanding of reaction rates, offering fascinating insights into the dynamic nature of chemical processes.
FAQs
Q: What is a zero-order reaction?
A: A zero-order reaction is a chemical reaction in which the rate remains constant and is independent of the concentration of reactants.
Q: Are zero-order reactions common?
A: Zero-order reactions are relatively rare compared to other types of reactions. They occur when the rate of reaction is not influenced by the concentration of the reactants.
Q: How can zero-order reactions be identified?
A: Zero-order reactions can be identified by plotting the concentration of reactants against time. If the graph is linear with a constant slope, it indicates a zero-order reaction.
Q: What are some real-life examples of zero-order reactions?
A: Examples of zero-order reactions include the decomposition of hydrogen peroxide, the degradation of alcohol in the human body, and the release of drugs from controlled-release systems.
Q: What are the implications of zero-order reactions in drug delivery?
A: Zero-order reactions are utilized in controlled drug release systems where a constant rate of drug release is required over a specific period of time.
Q: Can zero-order reactions be reversed?
A: Zero-order reactions are typically irreversible reactions. Reversing a zero-order reaction often requires altering the reaction conditions or introducing additional reagents.
Intrigued by the enigmatic world of chemical kinetics? Explore further and unravel the mysteries of reaction kinetics to gain a deeper understanding. Dive into the intricacies of rate law expressions and their significance in chemical reactions. Discover the power of catalysis and how it revolutionizes chemical processes. Embark on a captivating journey through the realms of chemical kinetics and expand your knowledge beyond zero-order reactions.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.


