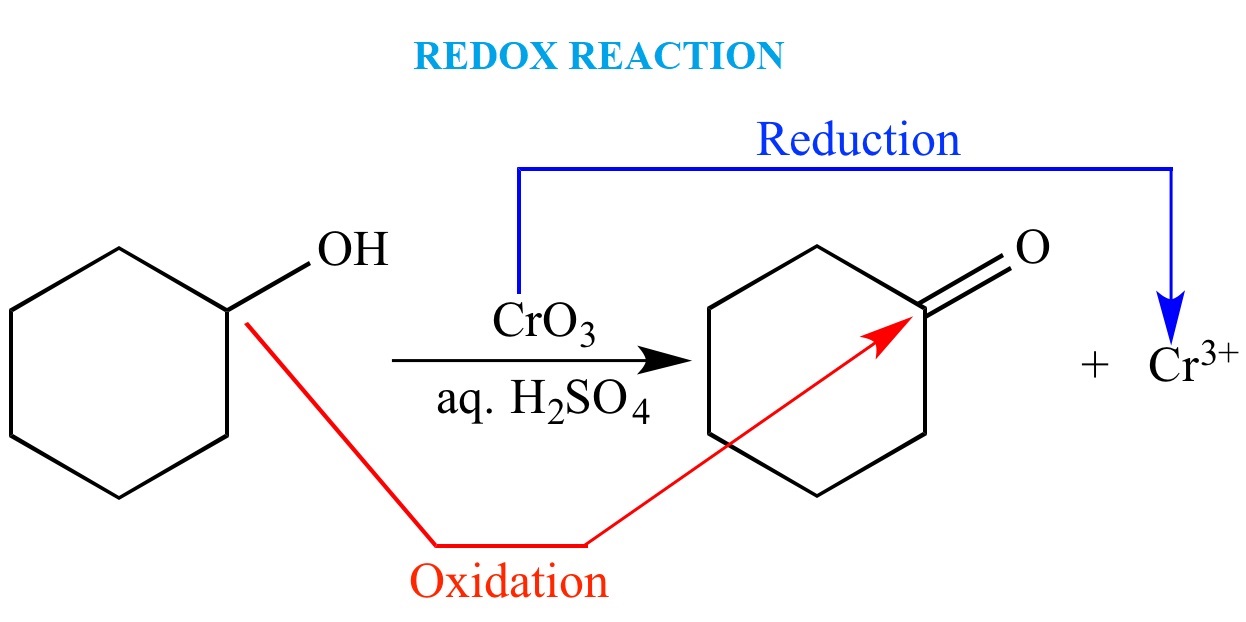
Redox reactions, short for reduction and oxidation reactions, are an intriguing and fundamental topic in the realm of chemistry. These reactions involve the transfer of electrons between chemical species, resulting in the alteration of their oxidation states. Redox reactions play a vital role in various natural processes and are a cornerstone in many industrial applications.
In this article, we will delve into the fascinating world of redox reactions and uncover 19 astonishing facts that will deepen your understanding of this essential chemical phenomenon. From the role of redox reactions in everyday life to their significance in environmental processes, prepare to be amazed by the incredible intricacies of redox chemistry.
Key Takeaways:
- Redox reactions are like a game of electron hot potato, where atoms and molecules pass around electrons to create energy, rust, and even help develop photographs!
- From batteries to biology, redox reactions are the MVPs of chemistry, powering everything from energy storage to environmental cleanup and even shaping the Earth’s crust.
Redox Reactions: The Cornerstone of Chemistry
Redox reactions, short for reduction-oxidation reactions, are fundamental to the study of chemistry. They involve the transfer of electrons between chemical species, resulting in changes in their oxidation states. These reactions play a crucial role in various natural processes and technological applications.
What’s in a Name?
The term “redox” is derived from the combination of two chemical processes: reduction and oxidation. Reduction occurs when a species gains electrons, while oxidation involves the loss of electrons. Together, these reactions create a dynamic interplay of electron transfer.
Electrons on the Move
Redox reactions involve the movement of electrons from one species to another. Electrons are negatively charged particles that orbit the nucleus of an atom. During a redox reaction, electrons are transferred between atoms, ions, or molecules, leading to a change in their overall charge.
Role of Redox Reactions in Energy Conversion
Redox reactions are at the heart of energy conversion processes, such as combustion, respiration, and photosynthesis. These reactions facilitate the release or absorption of energy by transferring electrons between reactants and products.
Rusting: A Familiar Redox Reaction
One common example of a redox reaction is the process of rusting, which occurs when iron reacts with oxygen in the presence of water. The iron undergoes oxidation, losing electrons and forming iron(III) oxide, while the oxygen is reduced, gaining electrons.
Balancing Redox Equations
Like any chemical reaction, redox reactions need to be balanced. This involves ensuring that the number of electrons lost in the oxidation half-reaction is equal to the number of electrons gained in the reduction half-reaction.
Redox Reactions in Batteries
Batteries are devices that rely on redox reactions to store and release electrical energy. For example, in a traditional alkaline battery, the anode undergoes oxidation, releasing electrons, while the cathode experiences reduction, accepting those electrons.
Redox Signaling in Biological Systems
Redox reactions play a crucial role in cellular signaling pathways, regulating important physiological processes. These reactions involve the transfer of electrons between molecules, leading to the activation or deactivation of specific biochemical pathways.
Ozone Formation in the Stratosphere
Redox reactions are responsible for the formation and destruction of ozone in the Earth’s stratosphere. In this process, oxygen molecules (O2) undergo a series of reactions involving the transfer of electrons, leading to the creation of ozone (O3).
Redox Reactions in Metabolism
Metabolism, the sum of all chemical reactions within a living organism, relies heavily on redox reactions. These reactions are involved in breaking down food molecules, releasing energy, and producing essential molecules for cellular function.
Redox Reactions in Corrosion
Corrosion, the process of material degradation, often involves redox reactions. For example, the exposure of metals to moisture and oxygen leads to oxidation, resulting in the formation of metal oxides and the deterioration of structures.
Redox Reactions in Environmental Remediation
Redox reactions play a crucial role in environmental cleanup processes, such as the decomposition of pollutants. These reactions can break down harmful substances into less toxic forms, helping to mitigate the impact of pollution on ecosystems.
Redox Reactions in Medicine
Redox reactions are important in medicine, particularly in the context of antioxidants and free radicals. Antioxidants help prevent damage to cells by reducing harmful free radicals through redox reactions, which stabilize these highly reactive molecules.
Redox Reactions in Photography
In traditional photography, redox reactions are involved in the development process. The reduction of silver ions to metallic silver and the oxidation of developing agents allow for the formation of an image on photographic film or paper.
Redox Reactions and Fuel Cells
Fuel cells utilize redox reactions to generate electrical energy. Hydrogen fuel cells, for example, involve the oxidation of hydrogen at the anode and the reduction of oxygen at the cathode, with the flow of electrons producing electrical power.
Redox Reactions and Environmental Redox Potential
Environmental redox potential is a measure of the electron-accepting or electron-donating capacity of a system. Redox reactions occurring in natural environments influence the redox potential, which, in turn, affects microbial processes and nutrient cycles.
Redox Reactions in Industrial Processes
Redox reactions are essential in numerous industrial applications. They are used in chemical synthesis, the production of metals, electroplating, wastewater treatment, and many other processes that rely on electron transfer.
Redox Reactions and Energy Storage
Efficient energy storage is a crucial aspect of renewable energy systems. Redox flow batteries, which store energy in the form of reversible redox reactions, have emerged as a promising solution for large-scale energy storage.
Redox Reactions in Geology
Redox reactions have significant implications in geology, shaping the Earth’s crust and influencing the distribution and concentration of minerals. These reactions play a vital role in soil formation, weathering, and the formation of ore deposits.
Conclusion
Redox reactions are a fascinating aspect of chemistry that play a crucial role in various biological, industrial, and environmental processes. From its involvement in combustion and corrosion to its significance in cellular respiration and photosynthesis, redox reactions are constantly shaping our world.
Throughout this article, we have explored 19 astonishing facts about redox reactions. We’ve learned about oxidation and reduction, the key players in these reactions, and how they are connected with electron transfer. We’ve also discovered how redox reactions are essential for the generation of electricity, the extraction of metals, and the functioning of batteries.
Furthermore, we’ve delved into the importance of redox reactions in reactivity series, titrations, and oxidation states. We’ve explored how redox reactions occur in our bodies, regulating vital processes such as metabolism and immune responses.
In conclusion, redox reactions are not merely an abstract concept studied in chemistry classrooms. They have real-world applications that impact our daily lives. By understanding the principles behind redox reactions, we gain insights into various fields of science and engineering, leading to new advancements and innovations.
FAQs
1. What is a redox reaction?
A redox reaction, short for reduction-oxidation reaction, is a chemical reaction where electrons are transferred between molecules, resulting in the change of oxidation states of the elements involved.
2. What is oxidation and reduction?
Oxidation refers to the process where an element loses electrons or gains oxygen. Reduction, on the other hand, involves the gain of electrons or the loss of oxygen by an element.
3. Can you provide an example of a redox reaction?
One common example of a redox reaction is the rusting of iron. Here, iron reacts with oxygen in the presence of moisture, resulting in the formation of iron(III) oxide.
4. How are redox reactions important in everyday life?
Redox reactions are essential for various everyday processes, such as combustion, respiration, and photosynthesis. They play a crucial role in energy production, metal extraction, and even in the functioning of batteries.
5. How do redox reactions occur in the human body?
In the human body, redox reactions are involved in metabolic processes, breaking down food molecules to release energy. They also play a vital role in immune responses, helping to neutralize harmful substances.
6. Are redox reactions reversible?
Yes, redox reactions can be reversible. They can proceed in both forward and backward directions, depending on the conditions and the availability of reactants and products.
7. Can redox reactions be balanced?
Yes, redox reactions can be balanced using the concept of stoichiometry. By ensuring that the number of atoms, charges, and electrons are conserved, a balanced equation can be obtained for the redox reaction.
8. Do all chemical reactions involve redox reactions?
No, not all chemical reactions involve redox reactions. There are other types of reactions, such as acid-base reactions and precipitation reactions, which do not involve electron transfer.
9. What are the practical applications of understanding redox reactions?
Understanding redox reactions has practical applications in various fields, such as environmental science, medicine, and energy production. It enables us to develop efficient catalysts, understand corrosion processes, and design better batteries and fuel cells.
10. How can I learn more about redox reactions?
There are many resources available to learn more about redox reactions. You can refer to chemistry textbooks, online educational websites, or consult with a chemistry teacher or tutor for further guidance.
Redox reactions play a crucial role in countless chemical processes, from the rusting of metal to the functioning of batteries. Understanding half-reactions, Nernst equations, and cell potentials can help you grasp the intricacies of these transformations. Explore the enigmatic world of half-reactions, captivating Nernst equations, and astonishing cell potentials to deepen your knowledge of redox reactions and their applications in various fields.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
