Transition metals are a fascinating group of elements that play a crucial role in various chemical processes. While many of us are familiar with some of the well-known transition metals like iron, copper, and gold, there are numerous surprising facts about this group that are often overlooked. From their unique electronic configurations to their ability to form complex compounds, transition metals offer a world of intrigue and possibilities. In this article, we will delve into 14 surprising facts about transition metals that will not only broaden your knowledge but also deepen your appreciation for the wonders of chemistry. So, buckle up and get ready to embark on a journey of discovery as we explore the lesser-known aspects of these remarkable elements.
Key Takeaways:
- Transition metals are versatile elements found in the middle of the periodic table, with high melting points and colorful compounds. They play vital roles in industries, biology, and even medicine, shaping our world in surprising ways.
- From vibrant gemstone colors to essential steel production, transition metals are crucial in various aspects of our lives. Their magnetic properties, catalytic abilities, and medical uses make them truly fascinating and impactful elements.
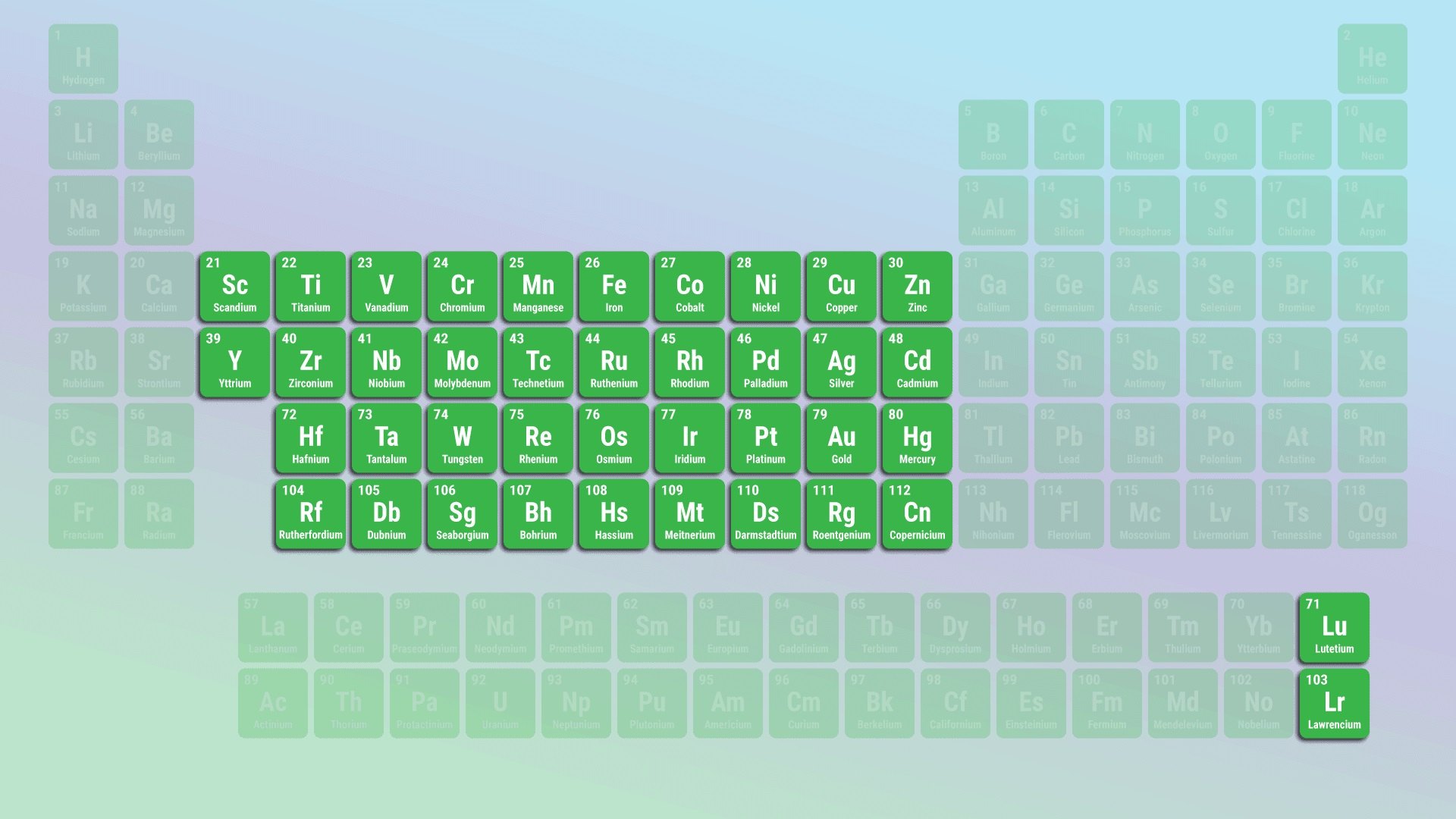
Transition metals are found in the middle of the periodic table.
As the name suggests, transition metals are located in the central portion of the periodic table. They span from group 3 to group 12, forming a bridge between the alkali metals and the metals in the post-transition and inner transition metal groups.
Transition metals have high melting and boiling points.
One remarkable characteristic of transition metals is their ability to withstand extreme temperatures. They have generally higher melting and boiling points compared to other elements, making them suitable for applications that involve extreme heat, such as in the aerospace and automotive industries.
Transition metals are excellent conductors of electricity.
Due to their delocalized electrons, transition metals exhibit great conductivity. This property makes them essential in the production of electrical wires, circuits, and other electronic components.
Transition metals can have multiple oxidation states.
Transition metals are known for their ability to exhibit multiple oxidation states, meaning they can gain or lose varying numbers of electrons. This versatility allows them to engage in a wide range of chemical reactions and form complex compounds.
Transition metals have colorful compounds.
Transition metal compounds often display vivid colors, which can be attributed to the presence of partially filled d-orbitals. These vibrant hues are commonly observed in pigments used in paints, dyes, and even fireworks.
Transition metals play a vital role in biological systems.
Many essential biological processes rely on transition metals. For instance, iron is crucial for oxygen transport in our blood, while copper is necessary for enzyme function. These elements are essential micronutrients for maintaining proper bodily functions.
Transition metals are used in catalysis.
Transition metals possess exceptional catalytic properties, making them indispensable in various industrial processes. They help facilitate reactions by lowering the activation energy, leading to more efficient production of chemicals and pharmaceuticals.
Transition metals have magnetic properties.
Several transition metals, such as iron, cobalt, and nickel, exhibit magnetic properties. This characteristic is utilized in the production of magnets for applications ranging from electronics to renewable energy systems.
Transition metals are crucial in the production of steel.
Steel, a vital material in construction and manufacturing, relies heavily on transition metals. Elements like iron, chromium, and nickel are added to create alloys, which enhance the strength, durability, and corrosion resistance of steel.
Transition metals are commonly used as catalysts in the petroleum industry.
The refining process of petroleum often relies on transition metal catalysts to convert crude oil into valuable products like gasoline, diesel, and various chemicals. These catalysts help increase efficiency and reduce environmental impact.
Transition metal ions are responsible for the vibrant colors in gemstones.
Transition metals contribute to the captivating colors seen in gemstones like emerald, ruby, and sapphire. The presence of specific transition metal ions within the crystal lattice gives each gemstone its unique hue.
Transition metals have been used for centuries in art and jewelry-making.
The beauty and versatility of transition metals have made them popular choices for artistic and decorative purposes. From ancient gold jewelry to modern sculptures, these elements have been cherished by artisans throughout history.
Transition metals have important applications in the field of medicine.
Transition metals play a significant role in medicinal chemistry, with many metal-based drugs used for the treatment of various diseases, including cancer. These compounds often exhibit targeted action and can be highly effective in combating certain illnesses.
Transition metals have isotopes with medical uses.
Isotopes of transition metals, such as technetium-99m, are widely utilized in medical imaging procedures. They emit gamma rays that aid in diagnosing conditions related to the heart, brain, bones, and other organs.
In conclusion, the 14 surprising facts about transition metals showcase the incredible diversity and importance of these elements in our daily lives. From their unique chemical properties to their wide-ranging applications, transition metals continue to shape our world in numerous ways.
Conclusion
In conclusion, transition metals are a fascinating group of elements with unique properties and applications. From their colorful compounds to their essential roles in biological processes, these metals play a crucial part in various fields, including chemistry, materials science, and medicine. Understanding the characteristics and behavior of transition metals allows scientists to develop new technologies, design innovative materials, and advance our understanding of the natural world.
Transition metals, such as iron, copper, and titanium, have proven to be versatile and valuable resources. Their ability to form stable complexes, participate in redox reactions, and exhibit catalytic activity makes them indispensable in industrial processes and everyday life. Whether it’s the bright colors produced by transition metal compounds or the role of iron in carrying oxygen within our bloodstream, these elements continue to astound us with their remarkable properties.
Now that you have learned about some surprising facts about transition metals, you can appreciate the importance and impact these elements have in our lives. From their contributions to technology and industry to their presence in our bodies, transition metals have a remarkable story to tell.
FAQs
Q: What are transition metals?
A: Transition metals are a group of metallic elements that occupy the central section of the periodic table. They are characterized by their ability to form stable complexes and exhibit variable oxidation states.
Q: How many transition metals are there?
A: There are 38 transition metals in total, including commonly known elements such as iron, copper, zinc, and gold.
Q: What are the unique properties of transition metals?
A: Transition metals have unique properties, including their colorful compounds, high melting points, malleability, and ability to act as catalysts in chemical reactions.
Q: What are the applications of transition metals?
A: Transition metals have a wide range of applications, such as in the production of steel, electronics, catalysts, and medical devices.
Q: Why are transition metals important in biological systems?
A: Transition metals play essential roles in biological systems, including oxygen transport, electron transfer, and enzymatic reactions.
Q: Can transition metals be toxic?
A: Some transition metals, such as mercury and lead, can be toxic to living organisms at high concentrations. However, many transition metals are essential for life in trace amounts.
Q: Are transition metals only found on Earth?
A: Transition metals are abundant on Earth, but they are also found in other celestial objects, such as meteorites and stars.
Transition metals' surprising properties merely scratch the surface of their elemental intrigue. Delving deeper into inner transition metals reveals even more enigmatic facts that will leave you astounded. Vanadium, a standout among transition metals, boasts an array of fun facts that showcase its unique qualities and applications. Ligand field theory provides a fascinating framework for understanding the complex interactions between transition metals and their surrounding molecules, offering enigmatic insights into their behavior and characteristics.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
