Welcome to the fascinating world of spectrophotometry! Whether you are a chemistry enthusiast, a researcher, or simply curious about the world around you, spectrophotometry is a topic that deserves your attention. In this article, we will delve into the intriguing realm of spectrophotometry and explore 19 captivating facts that will leave you amazed and inspired. From its historical roots to its modern applications, spectrophotometry has revolutionized the way scientists analyze and understand chemical substances. So, get ready to embark on a journey through the principles, techniques, and wonders of spectrophotometry. Let’s dive in and uncover the secrets behind this powerful analytical tool!
Key Takeaways:
- Spectrophotometry, invented in the 1940s, revolutionized chemistry by measuring light absorption to analyze substances’ concentration and composition, impacting industries from pharmaceuticals to forensics.
- From analyzing DNA to monitoring environmental pollutants, spectrophotometry’s future looks bright with emerging technologies expanding its applications in various fields, promising exciting advancements ahead.
The Invention of Spectrophotometry
One of the fascinating facts about spectrophotometry is that it was invented by Arnold O. Beckman in the early 1940s. His invention revolutionized the field of chemistry by allowing scientists to analyze the absorption and transmission of light in different substances.
The Working Principle of Spectrophotometry
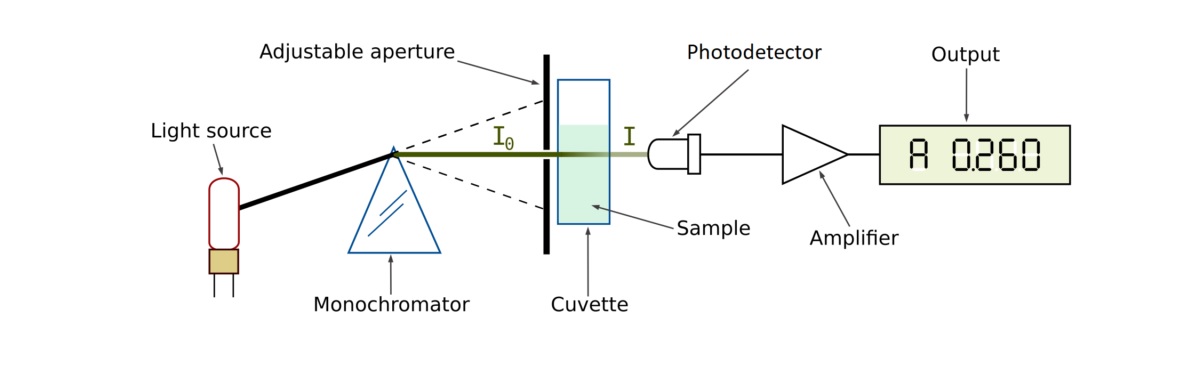
Spectrophotometry measures the amount of light that is absorbed or transmitted by a sample to determine its concentration or chemical composition. This technique is based on Beer-Lambert’s law, which states that the absorbance of a substance is directly proportional to its concentration.
Applications in Pharmaceutical Industry
Spectrophotometry plays a pivotal role in the pharmaceutical industry. It is used for drug discovery, quality control, and analysis of drug stability. By measuring the absorbance of a compound at different wavelengths, scientists can determine its purity and ensure its effectiveness.
Environmental Monitoring
Spectrophotometry is extensively utilized in environmental monitoring to analyze the concentration of pollutants in air, water, and soil. It helps scientists assess the impact of human activities on the environment and develop strategies for pollution control.
Forensic Analysis
Forensic scientists rely on spectrophotometry to analyze crime scene evidence. It helps in identifying and quantifying various substances such as drugs, blood, and DNA. This technique is instrumental in solving criminal cases and ensuring justice.
Food and Beverage Industry
In the food and beverage industry, spectrophotometry is used for quality control and determining nutritional content. It helps in detecting the presence of contaminants, measuring color intensity, and assessing the concentration of vitamins and minerals in food products.
Medical Diagnostics
Spectrophotometry plays a crucial role in medical diagnostics. It is used for the measurement of biomarkers, such as glucose and cholesterol levels, in blood samples. This aids in the diagnosis and monitoring of various diseases, including diabetes and cardiovascular disorders.
Ultraviolet-Visible Range Spectrophotometry
UV-Vis spectrophotometry is the most commonly used technique in spectrophotometric analysis. It involves the measurement of light in the ultraviolet and visible range (200-800 nm). This range allows researchers to analyze a wide range of substances and compounds.
Infrared Spectrophotometry
Infrared spectrophotometry, also known as IR spectroscopy, is utilized to study the molecular vibrations of substances. By measuring the absorption of infrared radiation, scientists can identify functional groups and determine the structure of organic compounds.
Raman Spectroscopy
Raman spectroscopy is an advanced technique that uses the scattering of light to provide information about molecular vibrations. It is used in various fields, including materials science, pharmaceuticals, and environmental analysis.
Quantitative Analysis
Spectrophotometry allows for quantitative analysis by measuring the absorbance or transmission of light. This data is then used to determine the concentration of a particular substance in a sample using calibration curves and mathematical calculations.
Qualitative Analysis
Aside from quantitative analysis, spectrophotometry is also valuable in qualitative analysis. By analyzing the absorption spectrum of a substance, scientists can identify unknown compounds based on their unique spectral patterns.
Visible Spectrophotometry
Visible spectrophotometry focuses on the measurement of light in the visible range (400-700 nm). This technique is particularly useful for analyzing colored compounds and assessing the concentration of pigments in samples.
Fluorescence Spectroscopy
Fluorescence spectroscopy is a powerful technique used in spectrophotometry to study the emission of fluorescence from a substance. It is widely used in biochemistry, molecular biology, and medical diagnostics.
Spectrophotometric Analysis of DNA
Spectrophotometry is extensively employed in the analysis of DNA. By measuring its absorbance at specific wavelengths, scientists can determine its concentration and purity, essential for various molecular biology techniques.
Spectrophotometric Colorimetry
Colorimetry is a branch of spectrophotometry that focuses on the measurement of color intensity. It is widely used in industries such as textiles, cosmetics, and printing to ensure consistency in color reproduction.
High-Performance Liquid Chromatography (HPLC)
HPLC is often coupled with spectrophotometry to enhance the analytical capabilities of both techniques. The combination allows for precise identification and quantification of compounds in complex mixtures.
Portable Spectrophotometers
Advancements in technology have led to the development of portable spectrophotometers. These compact devices provide ease of use and portability, making them ideal for on-site analysis in various industries.
The Future of Spectrophotometry
As technology continues to advance, so does spectrophotometry. New techniques, such as hyperspectral imaging and near-infrared spectroscopy, are emerging, expanding the applications of spectrophotometry in various fields.
Conclusion
In conclusion, spectrophotometry is a fascinating field that plays a crucial role in various scientific disciplines. The ability to measure the quantity of light absorbed or transmitted by a substance has revolutionized analytical techniques and has provided valuable insights into the properties of matter. From its early beginnings to the advanced spectrophotometric instruments available today, this technique has proven to be versatile and powerful in studying the structure and composition of substances.Through spectrophotometry, scientists have been able to explore the intricacies of chemical reactions, investigate the behavior of compounds in different environments, and even contribute to advancements in fields like medicine, environmental science, and materials science. The precision and accuracy of spectrophotometric measurements have made it an indispensable tool in scientific research and industrial applications.As technology continues to advance, spectrophotometry is expected to evolve further, opening new doors for discovery and innovation in the scientific community. From exploring new techniques and applications to refining existing methodologies, spectrophotometry will undoubtedly remain a cornerstone of scientific inquiry.
FAQs
1. What is spectrophotometry?
Spectrophotometry is a technique used to measure the amount of light absorbed or transmitted by a substance at different wavelengths.
2. How does spectrophotometry work?
It works by passing a beam of light through a sample and measuring the intensity of light that is absorbed or transmitted by the sample.
3. What are the applications of spectrophotometry?
Spectrophotometry is widely used in various fields such as chemistry, biochemistry, pharmaceuticals, environmental science, and forensic science for analysis and research purposes.
4. What are the benefits of using spectrophotometry?
Spectrophotometry provides accurate and precise measurements, allows for quantification of substances, and enables the identification and characterization of compounds.
5. What are the different types of spectrophotometers?
Some common types include UV-Vis spectrophotometers, infrared spectrophotometers, and fluorescence spectrophotometers.
6. Is spectrophotometry only used in laboratories?
No, spectrophotometry is also used in various industries for quality control, process monitoring, and product development.
7. Are spectrophotometers expensive?
The cost of spectrophotometers can vary, depending on the type and features. However, there are options available for different budget ranges.
8. Can spectrophotometry be used for quantitative analysis?
Yes, spectrophotometry is commonly used for quantitative analysis as it allows for the determination of the concentration of a substance in a sample.
9. Can spectrophotometry be used for qualitative analysis?
Yes, spectrophotometry can be used for qualitative analysis to identify unknown substances or determine the presence of specific functional groups.
10. How has spectrophotometry contributed to scientific advancements?
Spectrophotometry has contributed to advancements in various scientific fields, including drug discovery, environmental monitoring, and understanding molecular interactions.
Spectrophotometry has come a long way since its invention, revolutionizing various scientific fields. From pharmaceutical industry to environmental monitoring, forensic analysis to food and beverage industry, medical diagnostics to DNA analysis, this technique continues to evolve and shape our understanding of the world around us. As you explore the captivating world of spectrophotometry, don't forget to delve into the fascinating realm of analytical chemistry and unravel the mysteries of the electromagnetic spectrum.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
